
2,3-dihydropyrido[4,3-d]pyridazine-1,4-dione synthesis
- Product Name:2,3-dihydropyrido[4,3-d]pyridazine-1,4-dione
- CAS Number:31384-08-4
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
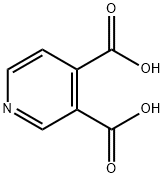
490-11-9
333 suppliers
$6.00/5g
![2,3-dihydropyrido[4,3-d]pyridazine-1,4-dione](/CAS/GIF/31384-08-4.gif)
31384-08-4
27 suppliers
$240.00/500 MG
Yield: 98.6%
Reaction Conditions:
Stage #1:3,4-pyridinecarboxylic acid with acetic anhydride for 1 h;Reflux;
Stage #2: with hydrazine hydrate for 4 h;Reflux;
Steps:
2,3-dihydropyrido[3,4-d]pyridazin-1,4-dione (1)
8.61 g (0.05 mol) 3,4-pyridinedicarboxylic acid wasadded to 30 mL (0.31 mol) acetic acid anhydride, and the mixture was heated to reflux and stirred for 1 h. After cooling coolingto room temperature, 30 mL hydrazine hydrate was added and the mixture was refluxed and stirred for 4 h.The product was collected by suction filtration, washedwith water and dried. Crystallized from ethanol/water toyield 98.6%; mp >300 °C; FTIR-ATR: 3404 (O-H), 3300-2200 (N-H), 1668 (C=O) cm-1; 1H NMR (DMSO-d6): δ9.34 (1H, s, H5), 9.03 (1H, d, J7-8 = 5.6 Hz, H7), 7.90(1H, d, J8-7 = 5.6 Hz, H8); HRMS calcd. for C7H6N3O2[M-H]+: 164.0460. Found: 164.0455. Anal. Calcd. forC7H5N3O2·1/3H2O: C, 49.71; H, 3.38; N, 24.84. Found: C,49.78; H, 3.27; N, 25.06%.
References:
Akçay, Sevilay;Ülger, Mahmut;Onurdağ, Fatma Kaynak;Dündar, Yasemin [Acta Chimica Slovenica,2018,vol. 65,# 4,p. 932 - 938]
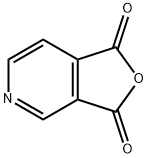
4664-08-8
138 suppliers
$6.00/250mg
![2,3-dihydropyrido[4,3-d]pyridazine-1,4-dione](/CAS/GIF/31384-08-4.gif)
31384-08-4
27 suppliers
$240.00/500 MG
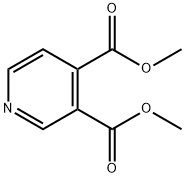
1796-83-4
56 suppliers
$60.00/10mg
![2,3-dihydropyrido[4,3-d]pyridazine-1,4-dione](/CAS/GIF/31384-08-4.gif)
31384-08-4
27 suppliers
$240.00/500 MG