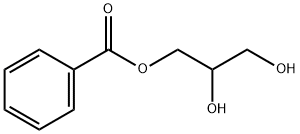
2,3-dihydroxypropyl benzoate synthesis
- Product Name:2,3-dihydroxypropyl benzoate
- CAS Number:3376-59-8
- Molecular formula:C10H12O4
- Molecular Weight:196.2
98760-27-1
0 suppliers
inquiry

3376-59-8
26 suppliers
$109.00/100mg
Yield:3376-59-8 98.7%
Reaction Conditions:
with hydrogenchloride in ethanol at 20; for 18 h;
Steps:
2j.B Step B: 2,3-dihydroxypropyl benzoate
The mixture of 20 g of the product from Step A (80 mmol, 1 eq.), 220 mL of a 1 N solution of HCl, and 220 mL of EtOH was stirred at rt for 18 h. After the reaction was quenched with cc. Na2CO3 and concentrated, the residue was extracted with EtOAc. The combined organic phases were washed with brine and dried to give 15.48 g (98.7%) of the desired product. 1H NMR (400 MHz, DMSO-d6) d ppm 8.00 (dd, 2H), 7.67 (tt, 1H), 7.54 (t, 2H), 5.03 (d, 1H), 4.71 (t, 1H), 4.30 (dd, 1H), 4.17 (dd, 1H), 3.79 (sx, 1H), 3.45 (m, 2H).
References:
WO2021/18857,2021,A1 Location in patent:Page/Page column 60-61
20834-13-3
0 suppliers
inquiry

3376-59-8
26 suppliers
$109.00/100mg