
(2,3-DIMETHOXY-PHENYL)-PYRIDIN-4-YL-METHANOL synthesis
- Product Name:(2,3-DIMETHOXY-PHENYL)-PYRIDIN-4-YL-METHANOL
- CAS Number:243640-27-9
- Molecular formula:C14H15NO3
- Molecular Weight:245.27
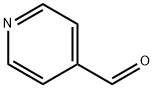
872-85-5
491 suppliers
$10.00/1g
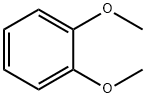
91-16-7
568 suppliers
$13.00/25g

243640-27-9
14 suppliers
$183.00/500mg
Yield:243640-27-9 81%
Reaction Conditions:
with n-butyllithium in hexane;water;toluene;
Steps:
48.c EXAMPLE 48c
EXAMPLE 48c Scheme F, step d: 4-[1-Hydroxy-1-(2,3-dimethoxyphenyl)methyl]pyridine (10) In a 2 L flask, 48 g of veratrole was dissolved in 200 g of toluene and 138 g of butyl lithium solution (15% in hexane) were added at temperatures from 4° C. to 6° C. The mixture was stirred for one hour at about 5° C. and 3 hours at ambient temperature. Then 26.7 g of 4-pyridinecarboxaldehyde (9) in 120 g of toluene was added at around ambient temperature. The reaction mixture was stirred for about 4.5 hours. The mixture was then cooled to around 5° C. and quenched with 133 mL of water. The mixture was then heated to around 80° C. The organic layer was separated, washed with 67 mL of water and separated. The residual water was removed by azeotropic distillation. The solution was then cooled to -15° C. to -5° C. The product was collected by filtration and washed with 27 g of cold toluene. The yield was 50 g of 4-[1-hydroxy-1-(2,3-dimethoxyphenyl)methyl]pyridine (10) (81% yield).
References:
US2002/151717,2002,A1
626-61-9
51 suppliers
$365.00/500mg
86-51-1
366 suppliers
$6.00/5g

243640-27-9
14 suppliers
$183.00/500mg

872-85-5
491 suppliers
$10.00/1g
2785-95-7
0 suppliers
inquiry

243640-27-9
14 suppliers
$183.00/500mg