2-(3-HYDROXY-3-METHYLBUTYL)PHENOL synthesis
- Product Name:2-(3-HYDROXY-3-METHYLBUTYL)PHENOL
- CAS Number:4167-73-1
- Molecular formula:C11H16O2
- Molecular Weight:180.24
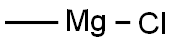
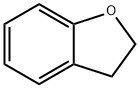
676-58-4
277 suppliers
$15.00/25ml
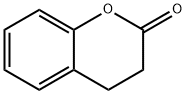
119-84-6
423 suppliers
$5.00/5g

4167-73-1
23 suppliers
$115.00/1 G
Yield: 100%
Reaction Conditions:
in tetrahydrofuran at 0 - 20;
Steps:
2-(3-Hydroxy-3-methylbutyl)phenol (7)
To a solution of dihydrocoumarin (5.0 g, 33.75 mmol) in anhydrous THF (50 mL) at 0 °C was added dropwise methylmagnesium chloride (101.1 mmol, 33.7 mL, 3.0 M in THF). The reaction mixture was slowly warmed to room temperature and stirred overnight. On completion, the reaction mixture was treated with ice chips containing 25 mL of 1N H2SO4. Extraction of the aqueous phase with Et2O, drying the latter over Na2SO4 and concentration under reduced pressure yielded the diol as a white solid (6.07 g, 100%): mp 110-112 °C; TLC Rf 0.37 (EtOAc : Hex, 1:1); 1H NMR (600 MHz, (CD3)2CO) δ 8.22 (broad s, 1H), 7.06-7.07 (m, 1H), 6.97 (m, 1H), 6.77-6.79 (m, 1H), 6.72 (m, 1H), 3.54 (s, 1H), 2.67-2.70 (m, 2H), 1.71-1.73 (m, 2H), 1.22 (s, 6H); 13C NMR (400 MHz, (CD3)2CO) δ 155.2, 129.9, 129.6, 126.8, 119.6, 115.2, 69.7, 44.1, 29.2, 25.1. Anal. (%) calcd for C11H16O2 C 73.30, H 8.95; found C 73.28, H 8.97.
References:
Jetson, Rachael;Malik, Neha;Luniwal, Amarjit;Chari, Venkatesh;Ratnam, Manohar;Erhardt, Paul [European Journal of Medicinal Chemistry,2013,vol. 63,p. 104 - 108] Location in patent:supporting information
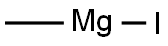
917-64-6
146 suppliers
$45.00/10ml

119-84-6
423 suppliers
$5.00/5g

4167-73-1
23 suppliers
$115.00/1 G
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4167-73-1
23 suppliers
$115.00/1 G
75-24-1
152 suppliers
$54.50/100ml

119-84-6
423 suppliers
$5.00/5g

4167-73-1
23 suppliers
$115.00/1 G