
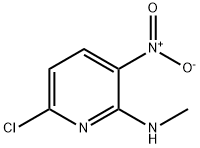
2,3-PYRIDINEDIAMINE, 6-CHLORO-N2-METHYL- synthesis
- Product Name:2,3-PYRIDINEDIAMINE, 6-CHLORO-N2-METHYL-
- CAS Number:89660-14-0
- Molecular formula:C6H8ClN3
- Molecular Weight:157.6
33742-70-0
96 suppliers
$102.00/1g

89660-14-0
30 suppliers
$215.00/100mg
Yield:89660-14-0 85%
Reaction Conditions:
with hydrogenchloride;dichloro-λ2-stannane dihydrate in lithium hydroxide monohydrate; for 18 h;Reflux;
Steps:
6-Chloro-N2-methylpyridine-2,3-diamine(10)
6-Chloro-N-methyl-3-nitropyridin-2-amine(9) (10.5 g, 56 mmol) and tin(II) chloride dihydrate (50.5 g,224 mmol) were suspended in concentrated HCl (80 mL) and refluxed for 18h. The solution was cooled to 0 °C and5M aqueous NaOH was cautiously added until the solution had a pH of 8. The mixture was subsequently partitioned with300 mL ethyl acetate and the layers were separated. The organics were washed with brine (200 mL),dried over sodium sulfate, filtered and concentrated in vacuo to give 6-chloro-N2-methylpyridine-2,3-diamine(10) as an off-white solid(7.5 g, 85%). HRMS m/z(M+H): calculated = 158.0480; observed = 158.0471.
References:
Layton, Mark E.;Reif, Alexander J.;Hartingh, Timothy J.;Rodzinak, Kevin;Dudkin, Vadim;Wang, Cheng;Arrington, Ken;Kelly, Michael J.;Garbaccio, Robert M.;O'Brien, Julie A.;Magliaro, Brian C.;Uslaner, Jason M.;Huszar, Sarah L.;Fillgrove, Kerry L.;Tang, Cuyue;Kuo, Yuhsin;Jacobson, Marlene A. [Bioorganic and Medicinal Chemistry Letters,2016,vol. 26,# 4,p. 1260 - 1264] Location in patent:supporting information