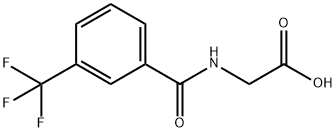
2-[3-(Trifluoromethyl)benzoyl]aminoacetic acid synthesis
- Product Name:2-[3-(Trifluoromethyl)benzoyl]aminoacetic acid
- CAS Number:17794-48-8
- Molecular formula:C10H8F3NO3
- Molecular Weight:247.17
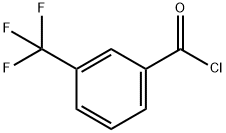
2251-65-2
213 suppliers
$10.00/1g
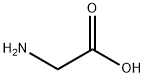
56-40-6
1205 suppliers
$5.00/25g
![2-[3-(Trifluoromethyl)benzoyl]aminoacetic acid](/CAS/GIF/17794-48-8.gif)
17794-48-8
120 suppliers
$20.00/100mg
Yield: 95%
Reaction Conditions:
Stage #1:3-(Trifluoromethyl)benzoyl chloride;glycine with sodium hydroxide in acetonitrile at 4 - 6; for 2.5 h;
Stage #2: with hydrogenchloride in water;acetonitrile; pH=3 at 0 - 6; for 1.5 h;
Steps:
71.A
A 12-L 4-neck round bottom flask equipped with a thermocouple controller, mechanical stirrer, heating mantle, condenser and a nitrogen in/outlet adapter was charged with gycine (1, Alfa Aesar) (318 g; 4.19 mol), acetonitrile (1.2 L), and a solution of sodium hydroxide (5.31 L; 10.62 mo) and the mixture was cooled to 4° C. with stirring. A solution of 3-(trifluoromethyl)benzoyl chloride (2, Alfa Aesar) (885.0 g; 4.12 mol) (640 mL) in acetonitrile (0.75 L) (total 1.39 L) was added dropwise over 2 h while the internal temperature was maintained between 4-6° C., and the slightly orange-pinkish solution was stirred at 4° C. for an additional 30 min. The reaction was acidified to pH=3 with conc. 37% HCl solution (400 mL added over 30 min) at 0-6° C., and stirred for 1 h at 0° C. (until a slightly yellowish suspension resulted). The solid was collected by filtration, washed with cold (0° C.) deionized ("D.I") H2O (300 mL*2), dried under air-suction for 2 h, and then placed in a drying oven at 60° C. under house vacuum (120 mmHg) for 20 h to afford pure 3 as an off-white solid. The filtrate was extracted with EtOAc (1 L*2), and the combined organic phases washed with brine (300 mL), and concentrated at 66° C. under house vacuum and then high vacuum (20 mmHg) to give crude product as an off-white waxy solid, which was triturated and sonicated with toluene (1 L) and stirred at 10° C. for 1 h. The resulting solid was collected by filtration, washed with hexanes (50 mL*2), dried in an vacuum oven at 50° C. under house vacuum to afford additional pure title compound, 3, as an off-white solid. The structure of 3 was confirmed with its 1H-NMR.
References:
Zhang, Xuqing;Hufnagel, Heather Rae;Hou, Cuifen;Johnson, Dana L.;Sui, Zhihua;Fegely, Barry;Breslin, David US2010/144695, 2010, A1 Location in patent:Page/Page column 61-62