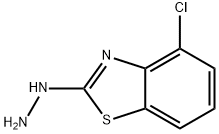
2(3H)-Benzothiazolone,4-chloro-,hydrazone(9CI) synthesis
- Product Name:2(3H)-Benzothiazolone,4-chloro-,hydrazone(9CI)
- CAS Number:51769-38-1
- Molecular formula:C7H6ClN3S
- Molecular Weight:199.66
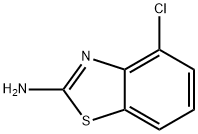
19952-47-7
212 suppliers
$18.00/5g

51769-38-1
15 suppliers
inquiry
Yield:51769-38-1 98%
Reaction Conditions:
with hydrogenchloride;hydrazine hydrate at 150; for 3 h;Inert atmosphere;
Steps:
4 Example 4: Preparation of 4-[1 -Aminomethylidenel-2-(4-chlorobenzothiazol-2-yl)-5- phenyl-2,4-dihvdropyrazol-3-one.
Example 4: Preparation of 4-[1 -Aminomethylidenel-2-(4-chlorobenzothiazol-2-yl)-5- phenyl-2,4-dihvdropyrazol-3-one. 4-Chlorobenzothiazol-2-ylamine (5.04g, 27.29mmol) was suspended in 35ml of ethylene glycol at room temperature under a nitrogen atmosphere. Hydrazine hydrate (3.98ml, 81.87mmol) was added followed by concentrated hydrochloric acid (2.24ml, 27.29mmol) and the resulting reaction mixture was heated on an oil bath to 150°C. After 1.5h a precipitate was formed and heating was continued for an additional 1.5h after which time the mixture was cooled, water was added and the resulting solids were filtered, washed with water and dried to give 5.33g (26.69mmol, 98%) of (4- chlorobenzothiazol-2-yl)-hydrazine. 1H-NMR {DMSO-d6): δ 9.40 (bs, 1 H), 7.62 (d, 1 H), 7.25 (d, 1 H), 6.95 (t, 1 H), 5.15 (bs, 2H). A solution of 1.09g (5.46mmol) of (4-chlorobenzothiazol-2-yl)-hydrazine and 1 .15g (6.01 mmol) of ethyl benzoylacetate in 30ml of ethanol was refluxed for 2 days under a nitrogen atmosphere. The reaction mixture was cooled and the precipitate was collected by filtration, washed with a little EtOH and dried to give 1 .56g (4.76mmol, 87%) of 2-(4-chlorobenzothiazol-2-yl)-5-phenyl-1 ,2-dihydropyrazol-3-one. 1H-NMR {DMSO-d6) δ 12.80 (bs, 1 H), 8.05 (d, 1 H), 7.85 (m, 2H), 7.60-7.40 (m, 4H), 7.35 (t, 1 H), 6.15 (s, 1 H). To a solution of 657mg (2.00mmol) of 2-(4-chlorobenzothiazol-2-yl)-5-phenyl-1 ,2- dihydro-pyrazol-3-one in 20ml of THF was added N,N-dimethylformamide dimethylacetal (293μΙ, 2.26mmol). The reaction was stirred over night at room temperature under a nitrogen atmosphere after which the solids were filtered off, washed with diethyl ether and dried to give 681 mg (1 .78mmol, 89%) of 2-(4- chlorobenzothiazol-2-yl)-4-[1 -dimethylaminomethylidene]-5-phenyl-2,4-dihydropyrazol- 3-one. 1H-NMR {DMSO-d6): δ 7.95 (d, 1 H), 7.60 (m, 3H), 7.55 (m, 4H), 7.25 (t, 1 H), 3.75 (s, 3H), 3.40 (s, 3H). A suspension of 545mg (1.42mmol) of 2-(4-chlorobenzothiazol-2-yl)-4-[1 - dimethylaminomethylidene]-5-phenyl-2,4-dihydro-pyrazol-3-one in 10ml 7N ammonia solution in MeOH was heated to 100°C in a pressure vessel for 18h. After cooling to room temperature, the solids were filtered, washed with a little EtOH and dried to give 460mg (1 .37mmol, 91 %) of 4-[1 -aminomethylidene]-2-(4-chlorobenzothiazol-2-yl)-5- phenyl-2,4-dihydropyrazol-3-one. 1H-NMR {DMSO-d6): δ 9.45 (bs, 2H), 8.00 (m, 2H), 7.75 (m, 2H), 7.50 (m, 4H), 7.35 (t, 1 H).
References:
WO2013/131931,2013,A1 Location in patent:Page/Page column 28; 29
95-51-2
370 suppliers
$5.00/5G

51769-38-1
15 suppliers
inquiry
608-31-1
299 suppliers
$12.00/25g

51769-38-1
15 suppliers
inquiry
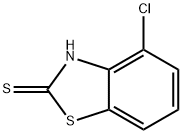
1849-65-6
63 suppliers
$10.00/250mg

51769-38-1
15 suppliers
inquiry
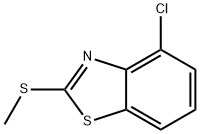
3507-40-2
6 suppliers
inquiry

51769-38-1
15 suppliers
inquiry