
2(3H)-Benzothiazolone,5-chloro-,hydrazone(9CI) synthesis
- Product Name:2(3H)-Benzothiazolone,5-chloro-,hydrazone(9CI)
- CAS Number:20174-72-5
- Molecular formula:C7H6ClN3S
- Molecular Weight:199.66
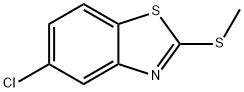
3507-41-3
63 suppliers
$45.00/50mg

20174-72-5
15 suppliers
inquiry
Yield:20174-72-5 95%
Reaction Conditions:
with hydrazine hydrate in ethanol at 100; for 3 h;Inert atmosphere;
Steps:
5 Example 5: Preparation of 4-[1 -Aminomethylidenel-2-(5-chlorobenzothiazol-2-yl)-5- phenyl-2,4-dihvdropyrazol-3-one.
5-Chlorobenzothiazole-2-thiol (5.21 g, 25.83mmol) was dissolved in 50ml DMF at room temperature under a nitrogen atmosphere. To the reaction mixture were added 4.28g (31 .OOmmol) of potassium carbonate and 1 .93ml (31 .OOmmol) of methyl iodide and stirring was continued over night after which time TLC (silica, 25% EtOAc in PE 40/60) indicated complete consumption of the starting material. Water was added to the reaction mixture and the resulting solids were filtered off, washed with water and dried to give 5.35g (24.80mmol, 96%) of 5-chloro-2-methylsulfanylbenzothiazole. 1H-NMR {DMSO-d6) δ 8.05 (d, 1 H), 7.90 (s, 1 H), 7.40 (d, 1 H), 2.75 (s, 3H). A mixture of 5-chloro-2-methylsulfanylbenzothiazole (5.03g, 23.32mmol) and hydrazine hydrate (1 1 .33ml, 233.17mmol) in 5ml of EtOH was heated under a nitrogen atmosphere to 100°C. After 3h a heavy precipitation was present in the reaction mixture after which time the suspension was cooled, water was added and the resulting solids were collected, washed with water and dried to give 4.43g (22.83mmol, 95%) of (5-chlorobenzothiazol-2-yl)-hydrazine. 1H-NMR {DMSO-d6): δ 9.10 (bs, 1 H), 7.65 (d, 1 H), 7.30 (s, 1 H), 6.95 (d, 1 H), 5.10 (bs, 2H). A solution of 935g (4.68mmol) of (5-chlorobenzothiazol-2-yl)-hydrazine and 990mg (5.15mmol) of ethyl benzoylacetate in 30ml of ethanol was refluxed over night under a nitrogen atmosphere. The reaction mixture was cooled and the precipitate was collected by filtration, washed with a little EtOH and dried to give 970mg (2.96mmol, 63%) of 2-(5-chlorobenzothiazol-2-yl)-5-phenyl-1 ,2-dihydropyrazol-3-one. 1H-NMR {DMSO-d6) δ 13.00 (bs, 1 H), 8.05 (d, 1 H), 7.90 (m, 3H), 7.50 (m, 3H), 7.40 (t, 1 H), 6.10 (s, 1 H). To a solution of 800mg (2.44mmol) of 2-(5-chlorobenzothiazol-2-yl)-5-phenyl-1 ,2- dihydro-pyrazol-3-one in 20ml of THF was added N,N-dimethylformamide dimethylacetal (357μΙ, 2.68mmol). The reaction was stirred over night at room temperature under a nitrogen atmosphere after which ether was added and the solids were filtered off, washed with diethyl ether and dried to give 790mg (2.06mmol, 85%) of 2-(5-chlorobenzothiazol-2-yl)-4-[1 -dimethylaminomethylidene]-5-phenyl-2,4- dihydropyrazol-3-one. 1H-NMR {DMSO-d6) δ 8.05 (d, 1 H), 7.85 (s, 1 H), 7.70 (s, 1 H), 7.60-7.40 (m, 5H), 7.35 (d, 1 H), 3.75 (s, 3H), 3.40 (s, 3H). A suspension of 650mg (1.70mmol) of 2-(5-chlorobenzothiazol-2-yl)-4-[1 - dimethylaminomethylidene]-5-phenyl-2,4-dihydropyrazol-3-one in 10ml 7N ammonia solution in MeOH was heated to 100°C in a pressure vessel for 24h. After cooling to room temperature, the solids were filtered, washed with a little EtOH and dried to give 439mg (1 .38mmol, 82%) of 4-[1 -aminomethylidene]-2-(5-chlorobenzothiazol-2-yl)-5- phenyl-2,4-dihydropyrazol-3-one. 1H-NMR {DMSO-d6) δ 9.50 (bs, 2H), 8.05 (m, 2H), 7.90 (s, 1 H), 7.75 (m, 2H), 7.50 (m, 3H), 7.40 (t, 1 H).
References:
WO2013/131931,2013,A1 Location in patent:Page/Page column 30
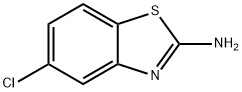
20358-00-3
159 suppliers
$6.00/250mg

20174-72-5
15 suppliers
inquiry

5331-91-9
160 suppliers
$37.00/5g

20174-72-5
15 suppliers
inquiry
29203-75-6
2 suppliers
inquiry

20174-72-5
15 suppliers
inquiry
108-42-9
465 suppliers
$10.00/5g

20174-72-5
15 suppliers
inquiry