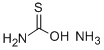2(3H)-Thiazolone, 5-acetyl-4-methyl- (9CI) synthesis
- Product Name:2(3H)-Thiazolone, 5-acetyl-4-methyl- (9CI)
- CAS Number:32497-14-6
- Molecular formula:C6H7NO2S
- Molecular Weight:157.1903
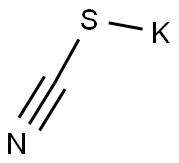
333-20-0
454 suppliers
$13.50/100G
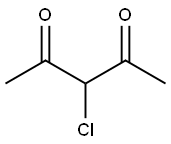
1694-29-7
109 suppliers
$40.00/10g

32497-14-6
13 suppliers
inquiry
Yield:32497-14-6 100%
Reaction Conditions:
Stage #1: potassium thioacyanate;3-chloropentane-2,4-dione in acetone at 0 - 20; for 6 h;
Stage #2: with hydrogenchloride in ethanol; for 14 h;Heating / reflux;
Steps:
2
A solution of potassium thiocyanate (5.67 g, 58 mmol) in Me2CO (45 mL) was cooled on an ice bath and 3-chloro-pentane-2, 4-dione (6.95 mL, 58 mmol) was added drop- wise. The mixture was warmed to room temperature and stirred for 6 h. After evaporation to dryness the residue was dissolved in EtOH (30 mL) and concentrated aq HC1 (15 mL) was added. This mixture was heated to reflux for 14 h. After cooling it was concentrated and the resulting precipitates were filtered and washed successively with cold MeOH and Et20 to afford the title compound as a tan solid (9.1 g, 100 %): mp 208-211 °C. Anal. RP-HPLC : tR 6.5 min (10-70 % MeCN over 20 min, purity 100 %). 1H-NMR (CDCl3) : No. 2. 33 (s, 3H, CH3), 2.38 (s, 3H, CH3), 11.9 (s, 1H, NH). 13C- NMR (DMSO-d6) : di 15.06, 29.94, 115.53, 142.99, 170.92, 189.91. FTIR: 3094,2850, 1669,1622, 1579 cm''. MS (ESt m/z 155.77 (M+H) +. Anal. (C6H7NO2S) C, H, N.
References:
WO2005/42525,2005,A1 Location in patent:Page/Page column 40
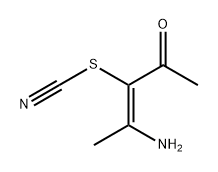
28465-07-8
0 suppliers
inquiry

32497-14-6
13 suppliers
inquiry
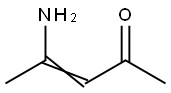
1118-66-7
124 suppliers
$5.00/250mg

32497-14-6
13 suppliers
inquiry
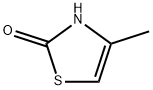
32497-10-2
34 suppliers
$45.00/2.5mg

75-36-5
587 suppliers
$17.92/100G

32497-14-6
13 suppliers
inquiry