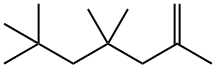
2,4,4,6,6-pentamethylhept-1-ene synthesis
- Product Name:2,4,4,6,6-pentamethylhept-1-ene
- CAS Number:14031-86-8
- Molecular formula:C12H24
- Molecular Weight:168.32
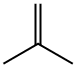
115-11-7
228 suppliers
$45.00/25g
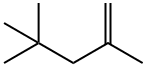
107-39-1
114 suppliers
$45.00/1g

14031-86-8
2 suppliers
inquiry
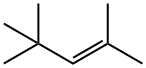
107-40-4
102 suppliers
$96.79/5g
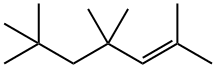
39761-68-7
1 suppliers
inquiry
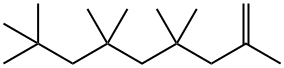
15796-04-0
2 suppliers
inquiry
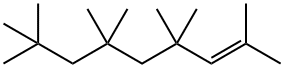
39761-73-4
0 suppliers
inquiry
Yield:-
Reaction Conditions:
H-SUZ-4 in butane,n- at 70; for 20 h;Product distribution / selectivity;
Steps:
1
The oligomerization of isobutene was carried out at 70 0C by using a fixed bed reactor containing 2 g of the H-SUZ-4(diameter: 0.2 - 1.0mm) that was obtained in the Example ofSynthesis and by flowing n-butane and isobutene (1:1 wt ratio) upward. The catalyst was pre-treated at 300 0C for 10 h by flowing nitrogen. The flow rates of hydrocarbons were controlled by mass flow controllers and the isobutene flow rate was adjusted for the isobutene WHSV (weight hourly space velocity) to be 10 h"1. The reaction temperature was maintained constant by using a liquid circulator. Circulated water at fixed temperature absorbs extra heat generated from the oligomerization. The isobutene conversion was calculated by measuring the total flow rates of n-butane and isobutene with mass flow meters. The isobutene conversion was re- checked by the analysis of gas-phase effluent by using a gas chromatography (GC) . The liquid product, after using a cold trap, was analyzed by a GC for the composition of dimers, trimers and tetramers. As shown in Table 1, the isobutene conversion, trimers selectivity and dimers selectivity were 99.9%, 68.8 wt% and 11.2 wt%, respectively, after the reaction time of 20 h. As shown in Fig. 2, the conversion and trimers selectivity were very high under the reaction
References:
WO2007/105875,2007,A1 Location in patent:Page/Page column 18

107-39-1
114 suppliers
$45.00/1g

14031-86-8
2 suppliers
inquiry