
2-[4-(4-fluorophenyl)piperazin-1-yl]ethan-1-ol synthesis
- Product Name:2-[4-(4-fluorophenyl)piperazin-1-yl]ethan-1-ol
- CAS Number:90096-38-1
- Molecular formula:C12H17FN2O
- Molecular Weight:224.27
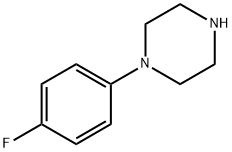
2252-63-3
207 suppliers
$13.43/250mgs:

540-51-2
237 suppliers
$17.00/5g
![2-[4-(4-fluorophenyl)piperazin-1-yl]ethan-1-ol](/CAS/20180527/GIF/90096-38-1.gif)
90096-38-1
6 suppliers
inquiry
Yield:90096-38-1 89%
Reaction Conditions:
with triethylamine in chloroform at 50;
Steps:
4.1.15. 2-(4-(4-Nitrophenyl)piperazin-1-yl)ethanol (24)
General procedure: A mixture of 1-(4-nitrophenyl)piperazine (5, 2.5 g, 12 mmol), 2-bromoethanol (1.28 mL, 18 mmol) and Et3N (1.6 mL, 18 mmol) wereheated at 50° C in CHCl3 (15 mL) for 30-35 h. Reaction mixturewasthen concentrated under reduced pressure and residue was dissolvedin EtOAc (15 mL). The organic layer was washed with water(10 mL x 3). The combined organic layer was then dried (anhyd.Na2SO4) and was concentrated under reduced pressure. The solidseparated out was washed with distilled hexane and again driedunder high vacuum. The pure yellow crystals were recrystallizedusing EtOAc/Hexane in excellent yield (yield 97%).
References:
Gupta, Sonal;Pandey, Deepti;Mandalapu, Dhanaraju;Sharma, Vikas;Shukla, Mahendra;Singh, Seema;Singh, Nidhi;Yadav, Santosh Kumar;Tanpula, Dilip Kumar;Singh, Surabhi;Maikhuri, Jagdamba P.;Shukla, Shubha;Lal, Jawahar;Siddiqi, Mohammad I.;Gupta, Gopal;Sharma, Vishnu L. [European Journal of Medicinal Chemistry,2017,vol. 132,p. 204 - 218]
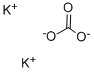
584-08-7
1192 suppliers
$5.00/100g

2252-63-3
207 suppliers
$13.43/250mgs:

540-51-2
237 suppliers
$17.00/5g
![2-[4-(4-fluorophenyl)piperazin-1-yl]ethan-1-ol](/CAS/20180527/GIF/90096-38-1.gif)
90096-38-1
6 suppliers
inquiry