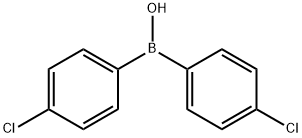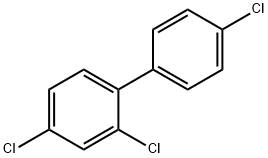
2,4,4'-TRICHLOROBIPHENYL synthesis
- Product Name:2,4,4'-TRICHLOROBIPHENYL
- CAS Number:7012-37-5
- Molecular formula:C12H7Cl3
- Molecular Weight:257.54
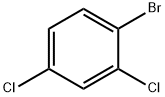
1193-72-2
265 suppliers
$9.00/5g
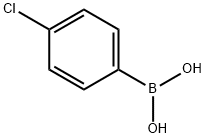
1679-18-1
589 suppliers
$10.00/1g

7012-37-5
52 suppliers
$131.00/2ml
Yield:7012-37-5 98%
Reaction Conditions:
with C20H29Cl2N5O2Pd;potassium carbonate in ethanol at 78 - 83; for 2.5 h;Suzuki Coupling;
Steps:
2,4,4'-Trichlorobiphenyl [44].
A 15 mL screw-top glass tube was loaded with 4 mL of 96% ethanol, 226 mg (1 mmol) of 1-bromo-2,4-dichlorobenzene, 172 mg (1.1 mmol) of 4-chlorophenylboronic acid, and 207 mg (1.5 mmol) of anhydrous K2CO3. The tube was put in an oil bath heated to 78°C. The reaction mixture was stirred during 3 min, and then 0.7 mg (0.0012 mmol) of acyclic diaminocarbene complex of palladium 7 in 0.5 mL of ethanol was added. The tube was tightly screwed and stirred during 2.5 h at 83°C. The mixture was then cooled to ambient, 10 mL of water was added, and the reaction product was extracted with a 2 : 1 hexane-dichloromethane mixture (320 mL), the organic layer was dried over anhydrous Na2SO4, the solvent was evaporated, and the residue was weighed and analyzed by 1H NMR and GLC. Yield 252 mg (98%), mp 56°C (methanol). 1H NMR spectrum, δ, ppm: 7.25 d (1, H6, J = 8.2 Hz), 7.32 d.d (1H, H5, J = 8.2, 2.0 Hz), 7.37 and 7.43 (4H, AA'XX' system, H2',3',5',6', J = 8.5 Hz), 7.51 d (1H, H3, J = 2.0 Hz).
References:
Kostenko;Eliseenkov;Petrov [Russian Journal of General Chemistry,2017,vol. 87,# 8,p. 1656 - 1662][Zh. Obshch. Khim.,2017,vol. 87,# 8,p. 1239 - 1246,8]
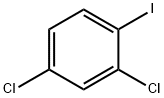
29898-32-6
141 suppliers
$6.00/1g

1679-18-1
589 suppliers
$10.00/1g

7012-37-5
52 suppliers
$131.00/2ml

876070-70-1
1 suppliers
inquiry

7012-37-5
52 suppliers
$131.00/2ml

29898-32-6
141 suppliers
$6.00/1g

7012-37-5
52 suppliers
$131.00/2ml