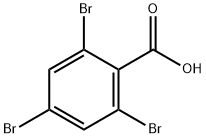
2,4,6-TRIBROMOBENZOIC ACID synthesis
- Product Name:2,4,6-TRIBROMOBENZOIC ACID
- CAS Number:633-12-5
- Molecular formula:C7H3Br3O2
- Molecular Weight:358.81
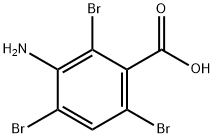
6628-84-8
25 suppliers
inquiry

633-12-5
89 suppliers
$25.00/250mg
Yield: 86%
Reaction Conditions:
with sulfuric acid;hypophosphorous acid;acetic acid;sodium nitrite in water at -5 - 20; for 12 h;
Steps:
1.2; 1.3; 1.4-2.2; 2.3; 2.4
(2) Dissolve intermediate I (110g, 0.29mol) in 2.2L glacial acetic acid.stand-by;Prepare a 10L glass reactor, add 330ml of pure water first, and after cooling to 5 ° C, slowly add 1.1L of 98% concentrated sulfuric acid, which has an exotherm and the internal temperature is controlled to drop below 10 ° C;After 1.5 drops are added, the ice-salt bath is cooled down to -5 ° C.Add sodium nitrite (60g, 0.87mol) in batches, exothermic, add 6g each time, after 30 minutes add sodium nitrite;Then control the internal temperature below -5 ° C, add 330ml 30% H3PO2 dropwise, the solution becomes cloudy;Finally, below -5 ° C,2.2L of intermediate glacial acetic acid solution prepared in advance was added dropwise. After the dropwise addition was completed, the mixture was stirred at room temperature for 12 hours for ripening;(3) After the reaction in step (2) is completed, steam distillation is performed to condense the acetic acid in the reaction solution to dryness; then the concentrated solution is cooled to 5 ° C, suction filtered, and the filtrate is discarded, and the filter cake is then subjected to pure water (4L × 3) Beating and washing, each stirring time is at least 1h, remove impurities, and finally suction filtration, drying,98 g of crude 2,4,6-tribromobenzoic acid was obtained;(4) Prepare a 1L glass flask, first add 500mL of toluene and 98g of crude 2,4,6-tribromobenzoic acid obtained in step (3), heat at 110 ° C and reflux, all the crystals are dissolved, and cool to -5 ° CAfter standing for 3 hours, crystallization, suction filtration, and vacuum drying at 50 ° C for 24 hours,89.1 g of white solid product 2,4,6-tribromobenzoic acid was obtained,The purity by HPLC was 99.5% and the yield was 86%.
References:
Shanghai Aladdin Biochemical Technology Co., Ltd.;Kan Hongzhu;Jiang Su;Xu Jiuzhen CN110437055, 2019, A Location in patent:Paragraph 0028; 0031-0037; 0039; 0042-0049
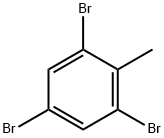
6320-40-7
179 suppliers
$20.00/5 g

633-12-5
89 suppliers
$25.00/250mg

626-39-1
481 suppliers
$5.00/5g

124-38-9
131 suppliers
$175.00/23402

633-12-5
89 suppliers
$25.00/250mg

124-38-9
131 suppliers
$175.00/23402
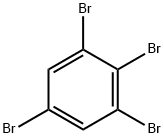
634-89-9
11 suppliers
inquiry

633-12-5
89 suppliers
$25.00/250mg
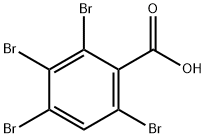
87976-24-7
1 suppliers
inquiry
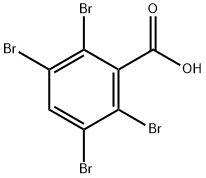
87976-25-8
0 suppliers
inquiry
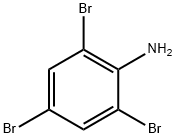
147-82-0
218 suppliers
$19.00/10g

633-12-5
89 suppliers
$25.00/250mg