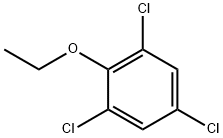
2,4,6-trichlorophenetole synthesis
- Product Name:2,4,6-trichlorophenetole
- CAS Number:23399-88-4
- Molecular formula:C8H7Cl3O
- Molecular Weight:225.5
Yield:23399-88-4 59%
Reaction Conditions:
with tetrafluoroboric acid dimethyl ether complex in dichloromethane; for 2 h;
Steps:
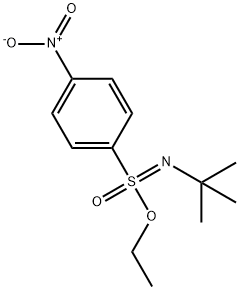
1-Bromo-4-ethoxybenzene; Typical Procedure for Reactions with Electron-Deficient Phenols
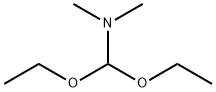
General procedure: Sulfonimidate 1 (299 mg, 1.04 mmol) and 4-bromophenol (129 mg, 0.746 mmol) were added to a magnetically stirred round-bottom flask fitted with a septum. Anhydrous CH2Cl2 (ca. 2 mL) was added, followed by HBF4·OMe2 catalyst (7.7 μL, 10 mol% of the amount of phenol) [TLC (hexane-EtOAc, 4:1 + 1% Et3N) monitoring]. After 50 min, when the reaction was complete (loss of TLC spot for 1), the catalyst was quenched with several dry molecular sieve pellets. Molecular sieves were filtered and rinsed with CH2Cl2, and the filtrate was concentrated by rotary evaporation. The concentrate was dissolved in anhydrous CH2Cl2 (1 mL) and loaded onto a flash chromatography column (silica gel, 5 g, hexane); yield: 81 mg (54%).
References:
Maricich, Tom J.;Allan, Matthew J.;Kislin, Brett S.;Chen, Andrea I-T.;Meng, Fan-Chun;Bradford, Christine;Kuan, Nai-Chia;Wood, Jeremy;Aisagbonhi, Omonigho;Poste, Alethea;Wride, Dustin;Kim, Sylvia;Santos, Therese;Fimbres, Michael;Choi, Dianne;Elia, Haydi;Kaladjian, Joseph;Abou-Zahr, Ali;Mejia, Arturo [Synthesis,2013,vol. 45,# 24,art. no. SS-2013-M0543-OP,p. 3361 - 3368]