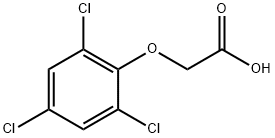
2,4,6-TRICHLOROPHENOXYACETIC ACID synthesis
- Product Name:2,4,6-TRICHLOROPHENOXYACETIC ACID
- CAS Number:575-89-3
- Molecular formula:C8H5Cl3O3
- Molecular Weight:255.48
Yield: 83%
Reaction Conditions:
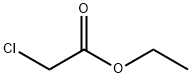
Stage #1:chloroacetic acid ethyl ester;2,4,6-Trichlorophenol with potassium carbonate;potassium iodide in N,N-dimethyl-formamide for 0.0666667 h;Microwave irradiation;
Stage #2: with sodium hydroxide in N,N-dimethyl-formamide for 0.0833333 h;Microwave irradiation;
Steps:
2.2.1. Synthesis of HCPA, HMCPA, and HTCPA
General procedure: The three ligands were prepared by microwave synthesis in the following route (Scheme 1). A mixture of 3-chlorophenol (5 mmol) for 1, 4-chloro-2-methylphenol (5 mmol) for 2 or 2,4,6-trichlorophenol (5 mmol) for 3, ethyl chloroacetate (5 mmol), K2CO3 (5 mmol), KI (1 mmol), PEG-600 (0.5 mmol), and DMF(2 mL) was irradiated in a microwave oven under 200 W for 4 min, then 5 mL of NaOH (2 M) was added and irradiated 5 min at 500 W. After cooling to room temperature, the resulting aqueous solution was acidified (pH 6) by the addition of a 1 M HCl solution under vigorous stirring. Column chromatography provided three white solids. 1H NMR (DMSO/TMS, 500 MHz, ppm): HCPA (Yield: 88%) δ 13.17 (s, 1H), 7.40 (t, J = 8.3 Hz, 1H), 7.14-7.07 (m, 2H), 7.02-6.97 (m, 1H), 4.83 (s, 2H). HMCPA (Yield: 86%) δ 13.04 (s, 1H), 7.22 (d, J = 2.2 Hz, 1H), 7.15 (dt, J = 10.3, 5.1 Hz, 1H), 6.83 (d, J = 8.8 Hz, 1H), 4.70 (d, J = 12.1 Hz, 2H), 2.18 (s, 3H). HTCPA (Yield: 83%) δ 13.25 (s, 1H), 7.81 (s, 2H), 4.68 (s, 2H).
References:
Xu, Xiuling;Yan, Saisai;Hu, Fan;Zhao, Wei;Shuai, Qi [Journal of Coordination Chemistry,2016,vol. 69,# 3,p. 541 - 550]
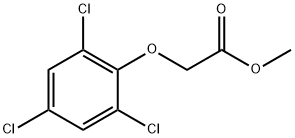
51550-51-7
2 suppliers
$57.00/100mg

575-89-3
64 suppliers
$64.00/5g
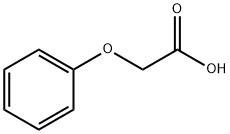
122-59-8
432 suppliers
$5.00/25g

575-89-3
64 suppliers
$64.00/5g