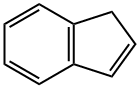
2,4,6-TRIMETHYL-1H-INDENE synthesis
- Product Name:2,4,6-TRIMETHYL-1H-INDENE
- CAS Number:150096-40-5
- Molecular formula:C12H14
- Molecular Weight:158.24
Yield: 96%
Reaction Conditions:
with sodium tetrahydroborate;magnesium sulfate in tetrahydrofuran;methanol
Steps:
B 2,4,6-Trimethylindene (2)
Example B 2,4,6-Trimethylindene (2) 20.4 g (117 mmol) of 2,5,7-trimethyl-l-indanone (1) were dissolved in 300 ml of a mixture of tetrahydrofuran/methanol (2:1), and 6.6 g (175 mmol) of NaBH4 were added at room temperature. The mixture was stirred for a further hour, 50 ml of half-concentrated HC1 were added and the mixture was extracted with ether. The combined organic phases were driedsodium sulfate and freed from the solvent. The residue was transferred to a distillation apparatus, and 13 g of magnesium sulfate were added. A vacuum of about 10mbar was applied and the mixture was heated up until the product distilled(130°-150° C). The distillation gave 17.7 g of the indene as a colorless oil. The yield was 96%. Mass spectrum: 158 M+, correct disintegration pattern.
References:
Hoechst Aktiengesellschaft US5374752, 1994, A

65001-59-4
29 suppliers
inquiry

150096-40-5
26 suppliers
inquiry
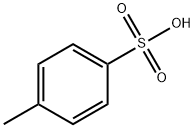
104-15-4
721 suppliers
$133.00/500g

150096-40-5
26 suppliers
inquiry

108-38-3
351 suppliers
$10.60/50gm:
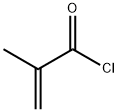
920-46-7
326 suppliers
$20.00/1g

150096-40-5
26 suppliers
inquiry
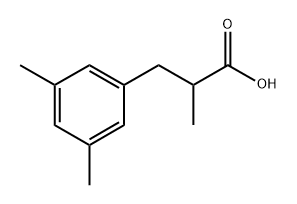
61227-50-7
4 suppliers
inquiry

150096-40-5
26 suppliers
inquiry