2,4,6-trimethylbenzene-1,3,5-tricarboxylic acid synthesis
- Product Name:2,4,6-trimethylbenzene-1,3,5-tricarboxylic acid
- CAS Number:18239-15-1
- Molecular formula:C12H12O6
- Molecular Weight:252.22

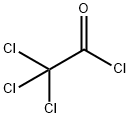
69125-03-7
6 suppliers
inquiry

18239-15-1
26 suppliers
inquiry
Yield:18239-15-1 53%
Reaction Conditions:
with chromium(VI) oxide;sulfuric acid;water in acetone at 0 - 30; for 1 h;
Steps:
4.4d
The oxidation reagent was prepared separately by portion wise addition of chromium (VI) oxide (21.4 g, 214 mmol) to a stirred solution of sulphuric acid (21.4 mL, 18 M). The now brown slurry was cooled in an ice bath and water (64 mL) was added slowly, forming a red solution. The chromium reagent was added drop wise to an ice cooled solution of compound 27 (5.0 g, 23.8 mmol) in acetone (278 mL). The reaction mixture was stirred at 0 0C for 20 min then allowed to attain room temperature during 30 minutes and then placed in an oil bath at 30 °C for 10 minutes. The reaction mixture was then crashed into water (550 mL), extracted with ether (200 mL) three times and the combined organic extracts were then washed with water (200 mL). The organic phase was dried with Na2SO4, filtered and evaporated to give crude 28 (4.2 g). The white crystals of crude 28 were suspended in water (70 mL) and the pH was adjusted to 7 by addition of NaOH (50 mL, IM). The now clear solution was passed through an ion exchange column (Dowex 50x8, dimensions: D: 3 cm, L: 7 cm) end eluted with water (150 mL). The eluent was lyophilized to give a white powder that was crystallized in refluxing acetic acid (100 mL). After cooling the crystals were filtered off and rinsed with acetic acid to give compound 28 as a white powder (3.2 g, 53%).
References:
WO2007/64227,2007,A1 Location in patent:Page/Page column 60
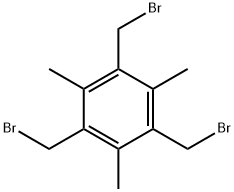
21988-87-4
124 suppliers
$25.00/1g

18239-15-1
26 suppliers
inquiry
1974-32-9
0 suppliers
inquiry

18239-15-1
26 suppliers
inquiry
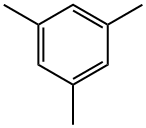
108-67-8
363 suppliers
$5.00/5G

18239-15-1
26 suppliers
inquiry