
2,4,6-TRIPHENYLPYRYLIUM CHLORIDE synthesis
- Product Name:2,4,6-TRIPHENYLPYRYLIUM CHLORIDE
- CAS Number:40836-01-9
- Molecular formula:C23H17ClO
- Molecular Weight:344.83
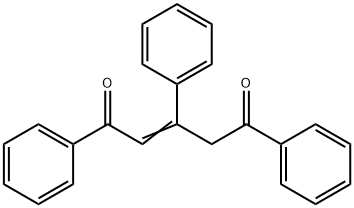
23800-57-9
2 suppliers
inquiry

40836-01-9
10 suppliers
inquiry
Yield:40836-01-9 85%
Reaction Conditions:
with hydrogenchloride in ethanol;lithium hydroxide monohydrate; for 0.5 h;Reflux;
Steps:
In the second step, the pseudobase (0.44 g, 1.35 mmol) was dissolvedin 50 mL of boiling ethanol. After dissolution was complete, 2.0 mL of37 % hydrochloric acid were added with vigorous stirring. The reactionmixture was then continually heated to refilux for 30 min. When thereaction ended, the solution was evaporated to give a crude yellowproduct. The product was recrystallized from methanol/diethyl ether. Itwas then dried in vacuum at 80 to obtain the pure product (0.40 g,1.16 mmol, yield 85 %). IR (cm 1): 3066, 1620, 1578, 1493, 1466,1447, 1420, 1277, 1246, 1192, 1030, 995, 772, 718, 679, 602. 1H NMR(CD3OD, 400 MHz, ppm) = 9.00 (2H, s,), 8.53 (6H, d, J = 7.6 Hz), 8.45(3H, t, J = 7.6 Hz), 7.88-7.75 (6H, m). 13C NMR (CD3OD, 100 MHz,ppm) = 171.1, 166.6, 135.0, 134.9, 133.0, 129.9, 129.8, 129.4, 129.2,128.4, 115.0. Anal. Calculated (Found) for C23H17OCl0.65H2O(356.55): C, 77.48 (77.23); H, 5.17 % (4.99 %).
References:
Bhowmik, Pradip Kumar;Principe, Ronald Carlo G.;Chen, Si L.;King, David;Han, Haesook;Jubair, Ahamed;Kartazaev, Vladimir;Gayen, Swapan Kumar [Chemical Physics Letters,2022,vol. 805,art. no. 139927]