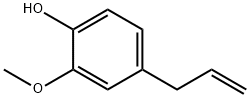
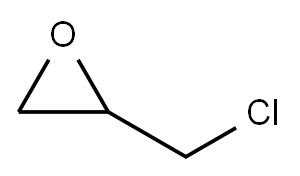
2-[(4-ALLYL-2-METHOXYPHENOXY)METHYL]OXIRANE synthesis
- Product Name:2-[(4-ALLYL-2-METHOXYPHENOXY)METHYL]OXIRANE
- CAS Number:36014-34-3
- Molecular formula:C13H16O3
- Molecular Weight:220.26
Yield:36014-34-3 92%
Reaction Conditions:
with sodium hydroxide in water at 85; for 0.5 h;
Steps:
2.1. Synthesis of 2-((4-allyl-2-methoxyphenoxy)methyl)oxirane (2)
The route followed for the synthesis of 3a-e is given in Scheme 1.Sodium hydroxide (0.24 g, 6.6 mmol) in water (10 mL) was added dropwise to a solution of eugenol (1.084 g, 6.6 mmol) and epichlorohydrin(10 mL) in 85 °C over a period of 0.5 h under vigorousstirring. The reaction mixture was kept at the same temperature till theeugenol completely consumed. Then the reaction mixture was solved inethyl acetate (20 mL) and washed with brine (2x15 mL), dried overMgSO4, and filtered. Organic phase was removed under reduced pressureand oily residue was obtained 2 (1.45 g, 92%).
References:
Gen? Bilgi?li, Hayriye;Kestane, Ali;Taslimi, Parham;Karabay, Oguz;Bytyqi-Damoni, Arlinda;Zengin, Mustafa;Gul?in, ?lhami [Bioorganic Chemistry,2019,vol. 88,art. no. 102931]