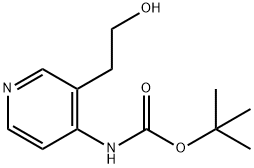
2-[4-(Boc-aMino)-3-pyridyl]ethanol synthesis
- Product Name:2-[4-(Boc-aMino)-3-pyridyl]ethanol
- CAS Number:219834-80-7
- Molecular formula:C12H18N2O3
- Molecular Weight:238.28
98400-69-2
172 suppliers
$6.00/1g

540-51-2
235 suppliers
$17.00/5g
![2-[4-(Boc-aMino)-3-pyridyl]ethanol](/CAS/GIF/219834-80-7.gif)
219834-80-7
25 suppliers
$165.19/100mg
Yield:219834-80-7 36%
Reaction Conditions:
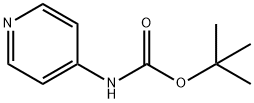
Stage #1: N-(4-pyridyl) t-butyl carbamatewith tert.-butyl lithium in tetrahydrofuran;pentane at -78 - -15; for 2.4 h;
Stage #2: 2-bromoethanolwith n-butyllithium in tetrahydrofuran;hexane;pentane at 20; for 2 h;
Steps:
9 EXAMPLE 9, METHODS
[00199] Synthesis of tert-butyl N~[3~(2-hydroxyethy])pyridm~4~y]j carbamate (11): Adapted from Spivey et at To a solution of 4-(Boc-amino)pyridme (1) (2.0 g, 10.3 mmol, 1 eq.) in 25 mL of dry THF at -78 °C was added /-Bu f .i (14.5 mL, 1.7 M, 24.7 mmol, 2.4 eq.) in pentane over 30 min. The resulting bright yellow suspension was stirred at -78 °C for 15 mi , at -15 °C for 2h and the recooled to -78 °C, I a separate flask, n-BuLi (7.38 mL, 2.5M. 18.54 mmol, 1 .8 eq) in hexanes was added to a solution of 2-bromocfhanol (1.294 mL, 15.45 mmol, 1.5 eq) in 20 mL of dry THF at -78 °C and stirred for 10 min. After 10 min, the bromoethanol solution was transferred "via cannula to the flask containing lithiated N-boc-4- aminopyridine over 10 min. The reaction was allowed to warm to room temperature and the mixture was stirred for 2h. The reaction was recooled to -78 °C and quenched with 5 ml, of water. The solution was partitioned between water (20 mL) and CH2CI2 (30 mL). The phases were separated and the extraction was completed with additional portions of CH2C12. The combined organic extracts were washed with brine, dried over MgS04 and evaporated under vacuum. The crude product was dissolved in a small amount of€{ purified by silica gel chromatography (EtOAc) to afford the product 11 (0.678 g, 36% yield). Rf = 0.15 (EtOAc). -Ν ΙΙ (500 MHz, CDCI3) δ: 1.51 (9H, s), 2.81 (2H, t, J ------ 5.0 Hz), 3.80 (1 H, br s), 3.94 (2H, t, J= 5.0 Hz), 7.92 (1H, d, J= 5.5 Hz), 8.15 (1H, s), 8.27 flH, d, J= 5.5 Hz), 8.63 (1H, s).
References:
WO2013/173746,2013,A2 Location in patent:Paragraph 00199
75-21-8
219 suppliers
$39.10/1mL

98400-69-2
172 suppliers
$6.00/1g
![2-[4-(Boc-aMino)-3-pyridyl]ethanol](/CAS/GIF/219834-80-7.gif)
219834-80-7
25 suppliers
$165.19/100mg