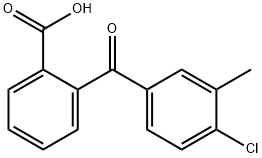
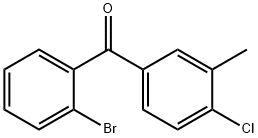
2-(4-CHLORO-3-METHYLBENZOYL)BENZOIC ACID synthesis
- Product Name:2-(4-CHLORO-3-METHYLBENZOYL)BENZOIC ACID
- CAS Number:141123-11-7
- Molecular formula:C15H11ClO3
- Molecular Weight:274.7
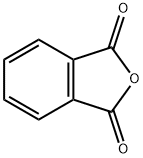
85-44-9
755 suppliers
$9.00/5g
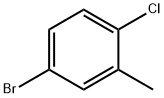
54932-72-8
209 suppliers
$6.00/1g

141123-11-7
14 suppliers
$387.00/1g
Yield:141123-11-7 50.4%
Reaction Conditions:
Stage #1: 2-chloro-5-bromotoluenewith n-butyllithium in tetrahydrofuran at -78; for 0.5 h;
Stage #2: phthalic anhydride in tetrahydrofuran at -78; for 2 h;
Steps:
To a stirring solution of 4-bromo-1-chloro-2-methyl-benzene 5 (4.92mL, 37.1mmol) in THF (75mL) cooled to -78°C was added n-butyllithium (14.9mL, 37.1mmol) dropwise. The solution was stirred at -78°C for 30min and then was added to a stirring solution of phthalic anhydride 6 (5.00g, 33.8mmol) in THF (90mL) at -78°C. The reaction was stirred at -78°C for 2h before being quenched with 1N HCl (100mL). The solution was allowed to warm to rt, the layers were separated and the aqueous layer was extracted with EtOAc (3×100mL). The combined organic layers were passed through a phase separator and concentrated. The crude material was purified using Teledyne ISCO Combi-Flash system (solid loading, 40G column, 40-80% EtOAc, 20min run) to give 2-(4-chloro-3-methyl-benzoyl)benzoic acid 7 (4.67g, 50.4% yield) as a white solid. 1H NMR (400MHz, CDCl3) δ (ppm): 8.10 (d, J=7.8Hz, 1H), 7.68 (dt, J=7.6, 1.2Hz, 1H), 7.64-7.62 (m, 1H), 7.59 (dt, J=7.9, 1.2Hz, 1H), 7.44 (dd, J=8.2, 2.2Hz, 1H), 7
References:
McGowan, Kevin M.;Nance, Kellie D.;Cho, Hykeyung P.;Bridges, Thomas M.;Jeffrey Conn;Jones, Carrie K.;Lindsley, Craig W. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 6,p. 1356 - 1359]
95-49-8
336 suppliers
$10.00/5g

141123-11-7
14 suppliers
$387.00/1g

74617-45-1
10 suppliers
$294.00/1g

141123-11-7
14 suppliers
$387.00/1g
951891-12-6
10 suppliers
$294.00/1g

141123-11-7
14 suppliers
$387.00/1g