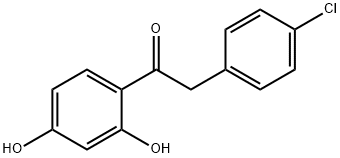
2-(4-Chlorophenyl)-1-(2,4-dihydroxyphenyl)ethanone synthesis
- Product Name:2-(4-Chlorophenyl)-1-(2,4-dihydroxyphenyl)ethanone
- CAS Number:15485-64-0
- Molecular formula:C14H11ClO3
- Molecular Weight:262.69
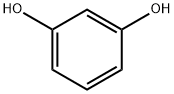
108-46-3
749 suppliers
$10.00/10g
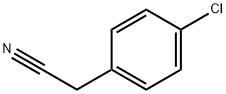
140-53-4
477 suppliers
$13.00/25g

15485-64-0
23 suppliers
$69.70/1g
Yield:15485-64-0 78%
Reaction Conditions:
Stage #1: recorcinol;p-chlorobenzyl cyanidewith hydrogenchloride;boron trifluoride diethyl etherate
Stage #2: with water at 80;
Steps:
1-(2,4-Dihydroxyphenyl)-2-(4-chlorophenyl)ethanone (1)
A mixture of 4-chlorophenylacetonitrile (15.1 g,0.1 mol) and resorcinol (11.0 g, 0.1 mol) in BF3Et2O (50 mL) was stirred for 4-6 h with purging by dry HCl, poured after12 h with stirring into H2O heated to 80°C, refluxed for 1.5-2 h, and cooled. The resulting precipitate was filtered off, rinsedwith cold H2O, and crystallized from MeOH. Yield 20.5 g (78 %), mp 153-154°, C14H11ClO3. 1 NMR spectrum(400 MHz., DMSO-d6, δ, ppm, J/Hz): 4.33 (2, s, 2), 6.27 (1, d, J = 2.4, H-3), 6.41 (1, dd, J = 8.8, 2.4, H-5), 7.31(2H, d, J = 8.8, -3′, 5′), 7.38 (2H, d, J = 8.8, -2′, 6′), 7.94 (1, d, J = 8.8, H-6), 10.72 (1, s, 4-), 12.42 (1, s, 2-).
References:
Garazd;Frasinyuk [Chemistry of Natural Compounds,2019,vol. 55,# 5,p. 813 - 817][Khim. Prir. Soedin.,2019,vol. 55,# 5,p. 702 - 705,4]
1878-66-6
446 suppliers
$6.00/25g

108-46-3
749 suppliers
$10.00/10g

15485-64-0
23 suppliers
$69.70/1g

108-46-3
749 suppliers
$10.00/10g

15485-64-0
23 suppliers
$69.70/1g

140-53-4
477 suppliers
$13.00/25g

15485-64-0
23 suppliers
$69.70/1g

25026-34-0
140 suppliers
$15.00/1g

108-46-3
749 suppliers
$10.00/10g

15485-64-0
23 suppliers
$69.70/1g