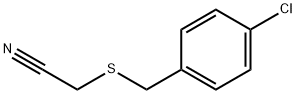
2-{[(4-chlorophenyl)methyl]sulfanyl}acetonitrile synthesis
- Product Name:2-{[(4-chlorophenyl)methyl]sulfanyl}acetonitrile
- CAS Number:21681-87-8
- Molecular formula:C9H8ClNS
- Molecular Weight:197.68
Yield:-
Reaction Conditions:
with potassium carbonate in 1,2-dimethoxyethane at 20; for 19 h;Reflux;
Steps:
Compound 4f:
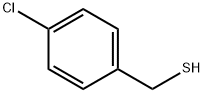
To a mixture of 4-chlorobenzenemethanethiol (540 μl, 4 mmol) and potassium carbonate (553 mg, 4 mmol) in dimethoxyethane (3 ml) was added chloroacetonitrile (255 μl, 4 mmol) dropwise. The resulting mixture was stirred at room temperature for one hour then refluxed for 18 hours. The solvent was removed under reduced pressure followed by the addition of water (3 ml). The mixture was extracted with diethyl ether (3 x 3 ml). The combined organic extracts were dried over anhydrous MgSO4 and concentrated. The crude oil was purified via silica gel flash chromatography (using 5% to 10% ethyl acetate in hexanes as the eluent). The p-chlorobenzylthioacetonitrile was isolated in (96% yield) and used directly in the subsequent step.
References:
Barker, Courtney A.;Allison, Sarah E.;Zlitni, Soumaya;Nguyen, Nick Duc;Das, Rahul;Melacini, Giuseppe;Capretta, Alfredo A.;Brown, Eric D. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 8,p. 2426 - 2431] Location in patent:supporting information