
2-(4-cyanophenyl)acetamide synthesis
- Product Name:2-(4-cyanophenyl)acetamide
- CAS Number:117753-06-7
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
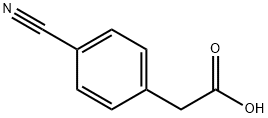
5462-71-5
136 suppliers
$8.00/250mg

117753-06-7
6 suppliers
inquiry
Yield:117753-06-7 350 mg
Reaction Conditions:
Stage #1: 4-cyanophenylacetic acidwith oxalyl dichloride;N,N-dimethyl-formamide in chloroform at 20; for 1 h;Inert atmosphere;Cooling with ice;
Stage #2: with ammonium hydroxide in tetrahydrofuran at 20; for 1 h;Inert atmosphere;Cooling with ice;
Steps:
2.1 2-(4-cyanophenyl)acetamide
Under a nitrogen atmosphere,(4-cyanophenyl) acetic acid (500 mg)In chloroform (6.0 mL) solution,Ice bath under cooling,Oxalyl chloride (620 mg) and N,After the addition of N- dimethylformamide (1 drop),And the mixture was stirred at room temperature for 1 hour.The reaction mixture was concentrated under reduced pressure.Residue of tetrahydrofuran (7.0 mL) ice bath under cooling to a suspension,28%After adding aqueous ammonia (3.00 mL),And the mixture was stirred at room temperature for 1 hour.Reaction solution of saturated sodium hydrogen carbonate aqueous solution was added, and the mixture was extracted with ethyl acetate. The organic layer was washed with a saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting solid was washed with diisopropyl ether to give the title compound (350 mg) as a pale yellow solid.
References:
JP2015/78127,2015,A Location in patent:Paragraph 0089
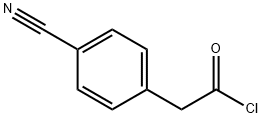
62044-37-5
2 suppliers
inquiry

117753-06-7
6 suppliers
inquiry

67-56-1
739 suppliers
$9.00/25ml

5462-71-5
136 suppliers
$8.00/250mg

117753-06-7
6 suppliers
inquiry
876-31-3
70 suppliers
$5.00/250mg

117753-06-7
6 suppliers
inquiry
874-86-2
267 suppliers
$11.00/5g

117753-06-7
6 suppliers
inquiry