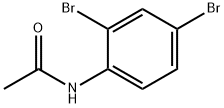
2',4'-DIBROMOACETANILIDE synthesis
- Product Name:2',4'-DIBROMOACETANILIDE
- CAS Number:23373-04-8
- Molecular formula:C8H7Br2NO
- Molecular Weight:292.96
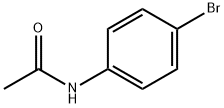
103-88-8
250 suppliers
$6.00/5g

23373-04-8
19 suppliers
$106.00/50mg
Yield:23373-04-8 96%
Reaction Conditions:
with hydrogen bromide;1-(chloromethyl)-4-fluoro-1,4-diazoniabicyclo-[2.2.2]octane bis(tetrafluoroborate) in lithium hydroxide monohydrate at 20; for 2.5 h;regioselective reaction;
Steps:
II. Bromination of anilides 1
General procedure: 1a as example: To a stirred suspension of N-(p-tolyl)acetamide 1a (75 mg, 0.5 mmol) and Selectfluor (213 mg, 0.6 mmol) in water (3.0 mL) was added HBr (40% aqueous, 0.08 mL, 0.55 mmol), and the mixture was stirred for 5 min at room temperature. After 1a was consumed, as indicated by TLC, the reaction mixture was quenched with saturated aqueous Na2S2O3 (2.0 mL) and water (20.0 mL), and extracted with CH2Cl2 (10.0 mL) three times. The residue obtained after evaporation of the solvent was purified by column chromatography on silica gel (petroleum ether-ethyl acetate = 6:1, v/v) to afford N-(2-bromo-4-methylphenyl)acetamide 2a as a white solid (108 mg, 95% yield).
References:
Liang, Deqiang;Li, Xiangguang;Wang, Chaowu;Dong, Qishan;Wang, Baoling;Wang, Hai [Tetrahedron Letters,2016,vol. 57,# 48,p. 5390 - 5394] Location in patent:supporting information
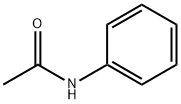
103-84-4
343 suppliers
$12.00/100g

23373-04-8
19 suppliers
$106.00/50mg

103-84-4
343 suppliers
$12.00/100g

103-88-8
250 suppliers
$6.00/5g

23373-04-8
19 suppliers
$106.00/50mg
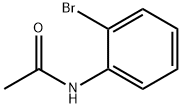
614-76-6
131 suppliers
$9.00/1g

23373-04-8
19 suppliers
$106.00/50mg
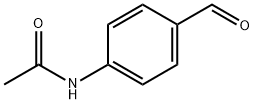
122-85-0
212 suppliers
$10.00/1g

23373-04-8
19 suppliers
$106.00/50mg