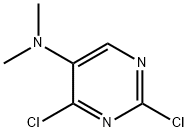
2,4-dichloro-N,N-dimethyl-pyrimidin-5-amine synthesis
- Product Name:2,4-dichloro-N,N-dimethyl-pyrimidin-5-amine
- CAS Number:5298-50-0
- Molecular formula:C6H7Cl2N3
- Molecular Weight:192.05

37454-51-6
16 suppliers
inquiry

5298-50-0
11 suppliers
inquiry
Yield: 68%
Reaction Conditions:
with trichlorophosphate for 2 h;Reflux;
Steps:
13
A solution of the aforementioned intermediate 2 (400 mg, 2.58 mmol) in POd3 (10 mL) was heated to reflux and stirred for 2 h. The mixture was cooled to room temperature and poured slowly into ice-water (100 mL). The aqueous phase was extracted with DCM (50 mL x 3). The organic layers were combined and dried over Na2SO4. The Na2SO4 was removed by filtration, and the volatileswere removed under reduced pressure, and the residue was purified by flashchromatography using a mixture of DCM and THF to give 2,4-dichloro-N,N-dimethylpyrimidin-5-amine 3 (340 mg, 68%) as a yellow oil. LRMS (M + H)m/z: calcd 192.00; found 192.
References:
WUSTROW, David;ZHOU, Han-Jie WO2015/89218, 2015, A1 Location in patent:Page/Page column 122