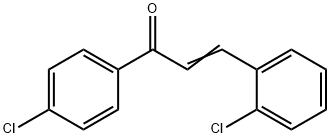
2,4'-DICHLOROCHALCONE synthesis
- Product Name:2,4'-DICHLOROCHALCONE
- CAS Number:19672-60-7
- Molecular formula:C15H10Cl2O
- Molecular Weight:277.15
Yield:19672-60-7 82%
Reaction Conditions:
with pyrographite;sodium hydroxide in water monomer at 70; for 6 h;Green chemistry;Aldol Condensation;
Steps:
Gene ral procedure for t he synthesis of compounds 4 mediated by AC
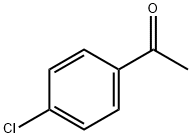
General procedure: AC 6 0 0 mg ) was added to a mix tu re of aldehyde 1 d 309 mg, 2 2 mmo l) and ketone 2 a 30 0mg, 2 0 mmo l) in a 1 0 mL f las k A soluti o n of Na O H ( 8 0 mg, 2 0 mmol) i n w ate r ( 2 .0 wasad ded and t he mixt ure w as stirr ed at 7 00 rpm at room temperature for 3 minutes . Af ter sti rri ng themi xture at 400 rpm for 6 h, CHCl 3 (10 mL) wa s added. A C was removed by filtration and washedwith CHCl 3 T h e mix tu r e was extracted w ith CHCl 3 twice . The combined ext ract s were washedwith wat er 4.0 mL × 3 )), d ried , a nd eva porated. T he res idue wa s p urified by colu m nc hrom a togr aphy o n silica gel using heptane ac etone 5 1) as el uent to give 4 k 441 mg, 81%).
References:
Tanemura, Kiyoshi;Rohand, Taoufik [Tetrahedron Letters,2021,vol. 71,art. no. 152918] Location in patent:supporting information