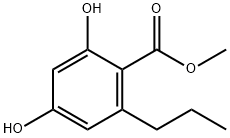
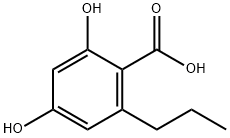
2,4-dihydroxy-6-propyl-benzoic acid methyl ester synthesis
- Product Name:2,4-dihydroxy-6-propyl-benzoic acid methyl ester
- CAS Number:55382-52-0
- Molecular formula:C11H14O4
- Molecular Weight:210.23
Yield:-
Reaction Conditions:
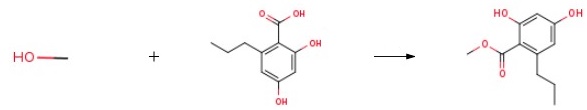
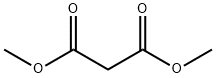
Stage #1: trans 3-hepten-2-one;malonic acid dimethyl esterwith sodium in methanol at 0; for 8 h;Reflux;
Stage #2: with bromine in N,N-dimethyl-formamide at 0 - 80; for 17 h;
Steps:
1B Example 1B
A solution of methanol (450 mL) at 0 °C was treated with sodium (25.5 g, 1 .1 1 mol) in portions and stirred until dissolved. Dimethyl malonate (143 mL, 1 .25 mol) was then added followed by (£)-hept-3-en-2-one (100 g, 0.89 mol) and the solution heated at reflux for 8 h. The methanol was removed then diluted with water (600 mL) and washed with CHCI3 (500 mL). The aqueous later was acidified and extracted with CHCI3 (3 x 400 mL). The combined organic layers were dried (MgS04) and concentrated to give a white solid. The white solid (5.37 g, 25.3 mmol) was dissolved in DMF (12 ml) and cooled to 0 °C. A solution of Br2 (1 .30 mL, 25.4 mmol) in DMF (6.6 mL) was slowly added and the solution stirred at 20 °C for 1 h. The solution was then heated to 80 °C for 16 h before cooling and treatment with 5% Na2S203 aqueous solution (200 mL) and being extracted with ethyl acetate (3 x 100 mL). The combined organic layers were dried (MgS04) and concentrated. The crude material was recrystallized from DCM/hexane to give a white solid
References:
WO2019/33168,2019,A1 Location in patent:Page/Page column 17
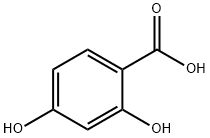
89-86-1
492 suppliers
$5.00/10g

55382-52-0
82 suppliers
inquiry

![3,9-Dioxa-2,10-disilaundeca-4,6-diene, 4-methoxy-2,2,10,10-tetramethyl-8-methylene-6-[(trimethylsilyl)oxy]-](/CAS/20210111/GIF/102342-54-1.gif)
