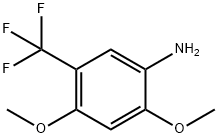
2,4-DIMETHOXY-5-(TRIFLUOROMETHYL)ANILINE synthesis
- Product Name:2,4-DIMETHOXY-5-(TRIFLUOROMETHYL)ANILINE
- CAS Number:228401-47-6
- Molecular formula:C9H10F3NO2
- Molecular Weight:221.18
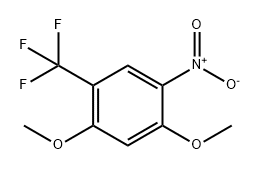
625119-46-2
0 suppliers
inquiry

228401-47-6
16 suppliers
$153.00/1g
Yield:228401-47-6 93%
Reaction Conditions:
with hydrogen;10% palladium on activated carbon in ethanol;ethyl acetate; under 2068.65 Torr; for 1 h;
Steps:
A.100 Example 100; N-[2,4-dimethoxy-5-(trifluoromethyl)phenyl]-N'-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]urea.
Sodium methoxide, prepared from sodium (0.92 g, 40.13 mmol) and 50 mL MeOH, is added dropwise to solution of 1,5-dichloro-2-nitro-4-(trifluoromethyl)benzene (5.0 g, 19.23 mmol) dissolved in 25 mL MeOH cooled to 0° C. The mixture is warmed to RT and stirred overnight. The mixture is heated under reflux for 8 h, then cooled to RT. An additional amount of sodium methoxide (19.23 mmol) is added and the reaction is heated under reflux for 4 h. The reaction is cooled, quenched with 1M citric and concentrated in vacuo. The residue is diluted in EtOAc and washed with 1M citric acid. The aqueous layer is extracted with EtOAc. The combined organic layers are washed with brine, dried (MgSO4), filtered and concentrated to provide 4.69 g (97% yield) of 1,5-dimethoxy-2-nitro-4-(trifluoromethyl)benzene. MS for C9H8F3NO4 (ESI) (M+H)+ m/z 252.1,5-Dimethoxy-2-nitro-4-(trifluoromethyl)benzene (4.24 g, 16.88 mmol) and a catalytic amount of 10% Pd/C (200 mg) are mixed in EtOH/EtOAc and shaken on a Parr hydrogenator apparatus in the presence of H2 (40 psi). After 1 h, the mixture is filtered through a pad of Celite. The solvent is removed in vacuo to afford 3.47 g (93% yield) of 2,4-dimethoxy-5-(trifluoromethyl)aniline as a light brown solid. MS for C9H10F3NO2 (ESI) (M+H)+ m/z 222.2,4-Dimethoxy-5-(trifluoromethyl)aniline (1.80 g, 8.14 mmol), dissolved in 50 mL EtOAc, is added dropwise over 1 h to excess phosgene (34.4 mL, 20% solution in toluene) dissolved in 30 mL EtOAc. The solution is heated under reflux for 30 min, cooled and concentrated in vacuo to provide 1.70 g (85% yield) of 1-isocyanato-2,4-dimethoxy-5-(trifluoromethyl)benzene. MS for C10H8F3NO3 (ESI) (M+H)+ m/z 248. Example 100 is obtained from 1-isocyanato-2,4-dimethoxy-5-(trifluoromethyl)benzene and 5-(trifluoromethyl)-1,3,4-thiadiazol-2-amine according to Method A, making non-critical changes. Yield 49%. HRMS (FAB) calculated for C13H10F6N4O3S+H 417.0456, found 417.0461.
References:
US2003/236287,2003,A1 Location in patent:Page 29, 30