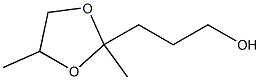
2,4-Dimethyl-1,3-dioxolane-2-(1-propanol) synthesis
- Product Name:2,4-Dimethyl-1,3-dioxolane-2-(1-propanol)
- CAS Number:36651-29-3
- Molecular formula:C8H16O3
- Molecular Weight:160.2108

5413-49-0
7 suppliers
inquiry
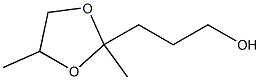
36651-29-3
0 suppliers
inquiry
Yield:36651-29-3 86%
Reaction Conditions:
with lithium borohydride in methanol;diethyl ether; for 2.5 h;Reflux;
Steps:
28
In a 5 L three-neck round bottom flask, 1,3-dioxolane-2-propanoic acid, 2,4-dimethyl-, ethyl ester, (264 grams, 1.31 moles) and methanol (79 mL) were dissolved into anhydrous diethyl ether (2.5 L). The flask was fitted with a mechanical stirrer, and placed into a heating mantle. While in the hood, lithium borohydride (43 grams, 1.97 moles) was added slowly. After addition of the lithium borohydride, the flask was fitted with two condensers, and heated at reflux for 2.5 hours. After heating, the reaction mixture was placed in an ice bath and with stiffing the reaction mixture was neutralized with 1M HCl. Additional water was added until all solids were dissolved. The ethereal layer was removed and the aqueous layer was extracted with dichloromethane (2×500 ml). The combined organic layers were dried over anhydrous sodium sulfate, and the solvents removed by rotary evaporator to yield pure product (181 gram, 86%). GC-MS and 1H-NMR data were obtained as shown in FIG. 3 to confirm the structure of the product and establish purity.
References:
US9458414,2016,B2 Location in patent:Paragraph 71

5413-49-0
7 suppliers
inquiry
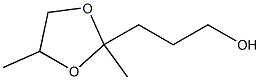
36651-29-3
0 suppliers
inquiry

57-55-6
1533 suppliers
$5.00/1g

71-36-3
1028 suppliers
$14.00/25mL