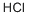
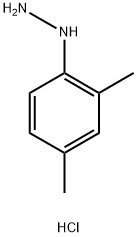
(2,4-dimethylphenyl)hydrazine synthesis
- Product Name:(2,4-dimethylphenyl)hydrazine
- CAS Number:615-00-9
- Molecular formula:C8H12N2
- Molecular Weight:136.19
95-68-1
313 suppliers
$14.00/5g

615-00-9
13 suppliers
inquiry
Yield:615-00-9 93%
Reaction Conditions:
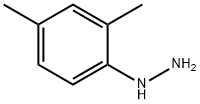
Stage #1: 2,4-Xylidinewith hydrogenchloride;sodium nitrite in water at -10;
Stage #2: with hydrogenchloride;tin(II) chloride hydrate in water at -10;
Stage #3: with sodium hydroxide in water; pH=14;
Steps:
1
Stage 1: 2,4-Dimethylhydrazine; Hydrochloric acid (89 g; 32% strength) and 40 ml of water are admixed with 43 g of 2,4-dimethylaniline, and the suspension is cooled to -5 to -10° C. and then admixed dropwise with a solution of 25 g of sodium nitrite and 104 ml of water, the aniline hydrochloride passing slowly into solution in the course of this addition. The clear, reddish brown solution is made nitrite-free with a little amidosulphonic acid. This diazonium salt solution, at a temperature of -10° C., is added rapidly dropwise to a solution of 200 g of SnCl2.H2O and 235 g of hydrochloric acid (32% strength). The resulting white suspension is stirred at this temperature for a number of hours and the tin double salt is isolated by suction filtration.; The tin double salt is added in a number of portions to an initial charge of 100 g of sodium hydroxide solution (45% strength) and 100 g of water, the pH is adjusted to 14 and the hydrazine is separated off by multiple MTBE extraction/ethyl acetate extraction from the aqueous phase. The solvent is distilled off under reduced pressure to give the hydrazine. Recrystallization from MTBE gives 45 g (93% of theory) of the pure 2,4-dimethylhydrazine.; M+: 136; 1H NMR (D6-DMSO): 6.85-6.97 (m, 3H), 3.7 (m, 3H), 2.25 (s, 3H), 2.1 (s, 3H)
References:
US2011/45104,2011,A1 Location in patent:Page/Page column 16-17
60480-83-3
164 suppliers
$10.00/500mg

615-00-9
13 suppliers
inquiry