
[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL synthesis
- Product Name:[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL
- CAS Number:885273-80-3
- Molecular formula:C10H8FNO2
- Molecular Weight:193.17
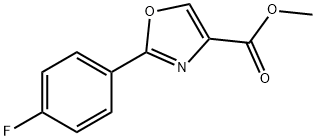
1065102-64-8
8 suppliers
inquiry
![[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL](/CAS/GIF/885273-80-3.gif)
885273-80-3
21 suppliers
inquiry
Yield:885273-80-3 86%
Reaction Conditions:
Stage #1: methyl 2-(4-fluorophenyl)-1,3-oxazole-4-carboxylatewith diisobutylaluminium hydride in tetrahydrofuran;toluene at -10 - 20; for 3 h;
Stage #2: with ammonium chloride in tetrahydrofuran;toluene;
Steps:
9
(2-(4-Fluorophenyl)oxazol-4-yl)methanolDIBAL-H (82 mL, 81.3 mmol, 1 M in toluene ) was added dropwise to a solution of methyl 2-(4-fluorophenyl)oxazole-4-carboxylate (6.0 g, 27.13 mmol) in dry THF (200 mL) at -10 °C. The reaction mixture was allowed to warm up to room temperature and stirred for 3 h. The reaction mixture was then quenched with saturated NH4CI solution, filtered through Celite, and extracted with EtOAc. The combined extracts were washed with 10% aqueous NaHC03 solution, H20 and brine, dried over anhydrous Na2S04, and concentrated under reduced pressure to get (2-(4-fluorophenyl)oxazol-4-yl)methanol (4.5 g, yield 86%) as a yellow solid. 1H NMR (300 MHz, CDCI3) δ 8.06 - 8.01 (m, 2H), 7.65 (s, 1 H), 7.18 - 7.12 (t, J = 8.7 Hz, 2H), 4.68 (s, 2H). MS (ESI) m/z: Calculated for C10H8FNO2: 193.05; found: 193.8 (M+H)+
References:
WO2011/88187,2011,A1 Location in patent:Page/Page column 52
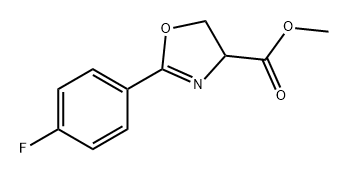
1314419-49-2
0 suppliers
inquiry
![[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL](/CAS/GIF/885273-80-3.gif)
885273-80-3
21 suppliers
inquiry
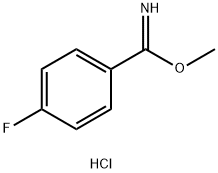
56108-05-5
4 suppliers
inquiry
![[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL](/CAS/GIF/885273-80-3.gif)
885273-80-3
21 suppliers
inquiry

459-57-4
582 suppliers
$6.00/25g
![[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL](/CAS/GIF/885273-80-3.gif)
885273-80-3
21 suppliers
inquiry
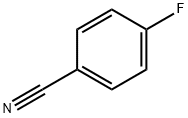
1194-02-1
503 suppliers
$6.00/10g
![[2-(4-FLUORO-PHENYL)-OXAZOL-4-YL]-METHANOL](/CAS/GIF/885273-80-3.gif)
885273-80-3
21 suppliers
inquiry