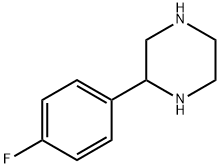
2-(4-FLUORO-PHENYL)-PIPERAZINE synthesis
- Product Name:2-(4-FLUORO-PHENYL)-PIPERAZINE
- CAS Number:65709-33-3
- Molecular formula:C10H13FN2
- Molecular Weight:180.22
403-32-7
35 suppliers
$63.61/250mgs:
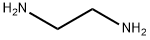
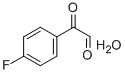
107-15-3
9 suppliers
$11.19/25ML

65709-33-3
76 suppliers
$43.00/1mg
Yield:65709-33-3 86%
Reaction Conditions:
Stage #1: (4-fluorophenyl)glyoxal;ethylenediamine in ethanol; for 4 h;
Stage #2: with sodium tetrahydroborate in ethanol at 20;
Steps:
42.1
A solution of ethylene diamine (7.4 g, 123.5 mmol) in ethanol (100 mL) was added dropwise over 15 minutes to a stirring solution of A- fluoroglyoxal (21.0 g, 123.5 mmol) in ethanol (300 mL) and the reaction was left for 4 hours. Sodium borohydride (23.5 g, 622 mmol) was added and the mixture was stirred overnight at room temperature. Water (200 mL) was added and the mixture was stirred for 1 hour after which the majority of the ethanol was removed in vacuo. The concentrated solution was extracted with DCM (4 x 100 mL) and the combined extracts were combined, washed with brine and dried over Na2SO4. The solvent was removed in vacuo to yield a pale yellow solid (19.0 g, 86%). 8.8 g of this material was dissolved in methanol (60 mL) and added to a solution of iV-acetyl-L-leucine (16.5 g, 95.2 mmol) in methanol (100 mL). Ethyl acetate (550 mL) was added and the mixture was left at room temperature overnight. The precipitate was filtered and dried to give a solid (9.0 g) which was taken up in 4M NaOH aq. (100 mL) and extracted with DCM (4 x 100 mL). The combined extracts were combined, washed with brine and the solvent was removed in vacuo to yield a solid (3.1 g). This solid was re-crystallized from EtOAc to yield 2.23 g of the S enantiomer (the title compound). The enantiomeric excess was determined by chiral chromatography employing the following chiral chromatographic conditions: Column: Phenomenex Chirex (S)-ICR 250 x 4.6 mm; solvent: n-heptane:ethanol [80:20] + 0.3% TFA; L = 254 nm, flow rate = 1 mL/min, UV sensitivity = 0.1 AUF; ~1 mg of compound in 1 mL of n-heptane: ethanol [75:25] using authentic chiral compounds and racemate as reference.
References:
WO2006/86705,2006,A1 Location in patent:Page/Page column 58