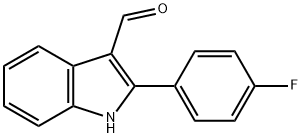
2-(4-FLUOROPHENYL)-1H-INDOLE-3-CARBOXAL& synthesis
- Product Name:2-(4-FLUOROPHENYL)-1H-INDOLE-3-CARBOXAL&
- CAS Number:70093-12-8
- Molecular formula:C15H10FNO
- Molecular Weight:239.24
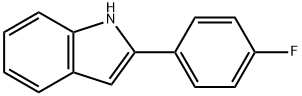
782-17-2
124 suppliers
$10.00/250mg

70093-12-8
28 suppliers
$45.00/50mg
Yield:70093-12-8 88%
Reaction Conditions:
Stage #1: 2-(4-fluorophenyl)indolewith N,N-dimethyl-formamide;trichlorophosphate at 10 - 50;
Stage #2: with sodium hydrogencarbonate in water at 60; for 0.25 h;
Steps:
9
1.7 mL of POCl3 was added drop wise to a solution of the 2(4-fluorophenyl)-indole (2.11 g) in 20 mL of DMF, at 10° C. The mixture was stirred at 50° C. for 1 hr, cooled and diluted with saturated NaHCO3. Th e suspension was heated at 60° C. for 15 min, cooled, and the precipitates of 2-(4-fluorophenyl)-indole-3-carboxaldehyde were filtered and crystallized from EtOH. Yield 2.10 g (88%).
References:
US2010/168417,2010,A1 Location in patent:Page/Page column 32-33
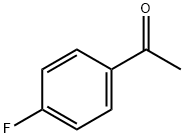
403-42-9
531 suppliers
$5.00/10g

70093-12-8
28 suppliers
$45.00/50mg
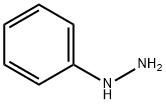
100-63-0
361 suppliers
$10.00/1g

70093-12-8
28 suppliers
$45.00/50mg
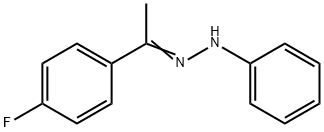
1994-40-7
0 suppliers
inquiry

70093-12-8
28 suppliers
$45.00/50mg