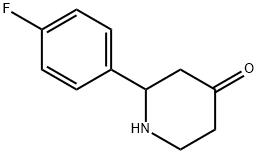
2-(4-fluorophenyl)piperidin-4-one synthesis
- Product Name:2-(4-fluorophenyl)piperidin-4-one
- CAS Number:414910-21-7
- Molecular formula:C11H12FNO
- Molecular Weight:193.22
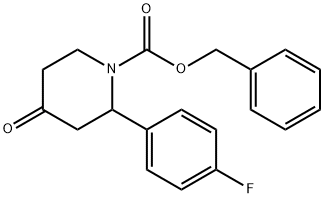
414910-20-6
6 suppliers
inquiry

414910-21-7
8 suppliers
inquiry
Yield:414910-21-7 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in tetrahydrofuran;ethyl acetate; under 760.051 Torr; for 12 h;
Steps:
19.3
A solution of benzyl 2-(4-fluorophenyl)-4-oxopiperidine- 1 -carboxylate (4.75 g, 14.50 mmol) in a mixture of ethyl acetate and tetrahydrofuran (1 : 1, 100 mL) was hydrogenated in the presence of 10% Pd/C at atmospheric pressure over 12h. The catalyst was filtered off and the filtrate was concentrated. The residue was dissolved in ethyl acetate (250 mL) and washed twice with saturated aqueous bicarbonate (100 mL), brine, then dried over anhydrous sodium sulfate, filtered and concentrated. The residue was dried to give 2-(4- fiuorophenyl)piperidin-4-one (2.8 g, quantitative). MS (EI) for CHHI2FNO: 194 (MH+).
References:
WO2010/138490,2010,A1 Location in patent:Page/Page column 37