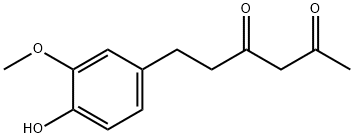
2,4-Hexanedione, 6-(4-hydroxy-3-methoxyphenyl)- synthesis
- Product Name:2,4-Hexanedione, 6-(4-hydroxy-3-methoxyphenyl)-
- CAS Number:113465-66-0
- Molecular formula:C13H16O4
- Molecular Weight:236.26
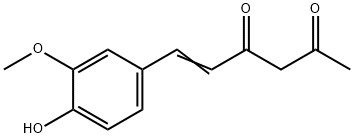
1924-25-0
1 suppliers
inquiry

113465-66-0
1 suppliers
inquiry
Yield:113465-66-0 89%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethyl acetate; for 2 h;
Steps:
7.3. Synthesis of DHFT
Feruloylacetone (1 mmol, 0.234 g) dissolved in 30 mL of ethyl acetate was hydrogenated over 20 mg of 10% Pd/C for 2 h. After the catalyst was filtered, and the solvent was evaporated under vacuum pressure, the residue was purified via column chromatography (silica gel, petroleum ether/ethanol = 50:1), 0.210 g of DHFT (pale yellow power) was obtained, yield 89%, m.p. 40-41 °C 1H NMR (300 MHz, CDCl3) δ: 2.03 (s, 3H, CH3CO-), 2.55 (t, J = 7.8 Hz, 2H, CH2CH2-), 2.86 (t, J = 7.8 Hz, 2H, CH2CH2-), 3.86 (s, CH3O-), 5.46 (s, 1H, CH-C), 5.49 (s, 1H, HO- in phenyl), 6.64 (d, J = 8.1 Hz, 1H, CHCH- in phenyl), 6.68 (s, 1H, -CHC in phenyl), 6.80 (d, J = 8.1 Hz, 1H, -CHCH in phenyl); 13C NMR (75 MHz, CDCl3) δ: 193.2, 191.1, 146.3, 143.9, 132.6, 120.7, 114.3, 110.9, 100.1, 55.8, 40.4, 31.2, 24.8.
References:
Feng, Jian-Ying;Liu, Zai-Qun [European Journal of Medicinal Chemistry,2011,vol. 46,# 4,p. 1198 - 1206] Location in patent:experimental part

121-33-5
1089 suppliers
$9.00/1g

113465-66-0
1 suppliers
inquiry
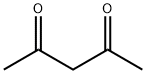
123-54-6
567 suppliers
$10.00/25ml

113465-66-0
1 suppliers
inquiry