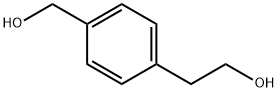
2-(4-(HYDROXYMETHYL)PHENYL)ETHANOL synthesis
- Product Name:2-(4-(HYDROXYMETHYL)PHENYL)ETHANOL
- CAS Number:4866-85-7
- Molecular formula:C9H12O2
- Molecular Weight:152.19
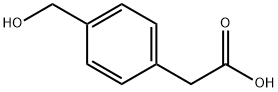
73401-74-8
188 suppliers
$14.56/1G

4866-85-7
26 suppliers
inquiry
Yield:4866-85-7 94%
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran at 0; for 3 h;Inert atmosphere;
Steps:
1 Preparation of Compound 10a:
4-(Hydroxymethyl)phenylacetic acid (3.5 grams, 21.1 mmol) was added to dry THF (100 mL) and set stirring under an argon atmosphere but with a vent. The reaction mixture was then cooled to 0 °C and then BH3.SMe2 (2M in THF, 37 mL, 73.7 mmol) was added at which point the solution began to effervesce; the vent and ice-bath were both maintained for the duration of the reaction. TLC analysis (ethyl acetate) indicated complete consumption of the starting material after 3 hours. The reaction mixture was quenched with IM HCl(aq) and then worked up with ethyl acetate and water. The organic layers were combined, dried with MgSO4 and then evaporated to yield 10a as a colorless oil (3.007 grams, 19.8 mmol, 94 %). (0526) 1 H NMR (300 MHz, CD3OD): δH = 7.27 (d, J = 8.2 Hz, 2H, Ar), 7.20 (d, J = 8.2 Hz, 2H, Ar), 4.56 (s, 2H, Ph-CH2OH), 3.73 (t, J = 7.1 Hz, 2H, CH2-CH2OH), 2.81 (t, J = 7.1 Hz, 2H, CH2CH2OH); (0527) ESI+ QTOFMS calculated for C9H11O ([M-OH]+) m/e 135.08; measured m/e 135.08.
References:
WO2022/153310,2022,A1 Location in patent:Page/Page column 12; 63; 64
52787-14-1
142 suppliers
$12.00/1g

4866-85-7
26 suppliers
inquiry
501-89-3
139 suppliers
$7.00/1g

4866-85-7
26 suppliers
inquiry