
2-(4-Iodo-phenoxy)-ethylaMine synthesis
- Product Name:2-(4-Iodo-phenoxy)-ethylaMine
- CAS Number:151978-97-1
- Molecular formula:C8H10INO
- Molecular Weight:263.08
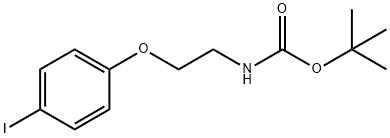
151978-98-2
4 suppliers
inquiry

151978-97-1
15 suppliers
$149.00/1g
Yield: 88%
Reaction Conditions:
with hydrogenchloride in diethyl ether;ethanol at 0 - 20; for 12 h;Concentration;Solvent;
Steps:
14.3.1 Step-3.i: Synthesis of2-(4-iodophenoxy)ethan- i-amine
10364] To a stirred solution of tert-butyl (2-(4-iodophe- noxy)ethyl)carbamate (25 g, 68.6 mmol, Example 3, Step-2) in ethanol (50 mE) was added at 0° C., 2M HC1 in ether (250 mE). The reaction mixture was stirred for 12 h at room temperature. After completion of reaction, reaction mixture was basified with saturated NaHCO3, extracted with 10% MeOR in DCM. Organic layer was concentrated under reduced pressure and the crude material was used in next step without further purification (16 g, 88%).
References:
Eisai R&D Management Co., Ltd.;BOCK, Mark;HAO, Ming-Hong;KORPAL, Manav;NYAVANANDI, Vijay Kumar;PUYANG, Xiaoling;SAMAJDAR, Susanta;SMITH, Peter Gerard;WANG, John;ZHENG, Guo Zhu;ZHU, Ping US2016/347717, 2016, A1 Location in patent:Paragraph 0363; 0364
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

151978-97-1
15 suppliers
$149.00/1g
88497-17-0
1 suppliers
inquiry
1517-05-1
105 suppliers
inquiry
540-38-5
335 suppliers
$10.00/1g

1972-28-7
378 suppliers
$15.00/5g

151978-97-1
15 suppliers
$149.00/1g
![1H-Isoindole-1,3(2H)-dione, 2-[2-(4-iodophenoxy)ethyl]-](/CAS/20180527/GIF/651330-71-1.gif)
651330-71-1
0 suppliers
inquiry

151978-97-1
15 suppliers
$149.00/1g

540-38-5
335 suppliers
$10.00/1g

151978-97-1
15 suppliers
$149.00/1g