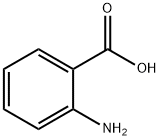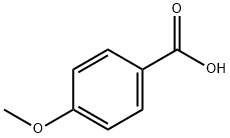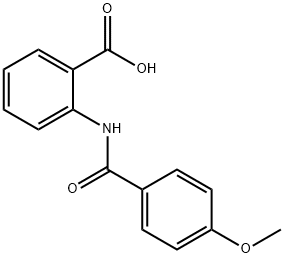
2-[(4-METHOXYBENZOYL)AMINO]BENZOIC ACID synthesis
- Product Name:2-[(4-METHOXYBENZOYL)AMINO]BENZOIC ACID
- CAS Number:34425-86-0
- Molecular formula:C15H13NO4
- Molecular Weight:271.27
Yield:34425-86-0 100%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 20;
Steps:
General procedure for the preparation of target compounds
General procedure: To a solution of anthranilic acid (1.1 mmol) in anhydrous THF (5 ml) was added 1.0 mmol of the appropriate benzoyl chloride at room temperature. After cooling the solution with ice bath, 1.2 mmol of triethylamine was added dropwise and reaction was stirred at room temperature for additional 3-10 h. The mixture was poured into a 25 ml cold solution of 1.0 M HCl, and precipitates were collected by filtration. Recrystallization from acetonitrile afforded the desired compound in quantitative yields. Data for 1H NMR spectra are reported as follows: chemical shift (multiplicity, coupling constants, and number of hydrogens). Abbreviations are as follows: s (singlet), d (doublet), t (triplet), m (multiplet). 1H NMR spectra were recorded on a Bruker Model Avance DMX 400 Spectrometer at 400 MHz. It is also recommended to acquire 1H NMR spectra in DMSO-d6, where otherwise exchangeable protons such as those of COOH, NH, and NH2 are usually visible. All chemical reagents and solvents were purchased from Merck or Aldrich Co. and used without further purification. Analytical grade solvents were used for the crystallization experiments. FT-IR spectra were measured with a Perkin-Elmer Model Bx 1600 instrument with the samples as KBr pellets in the 4000-400 cm- 1 range.
References:
Sen, Ibrahim;Kara, Hulya;Azizoglu, Akin [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2016,vol. 167,p. 50 - 58]
![methyl 2-[(4-methoxybenzoyl)amino]benzoate](/CAS/20180711/GIF/37619-28-6.gif)
37619-28-6
5 suppliers
inquiry
![2-[(4-METHOXYBENZOYL)AMINO]BENZOIC ACID](/CAS/GIF/34425-86-0.gif)
34425-86-0
13 suppliers
inquiry
5784-95-2
38 suppliers
$32.00/100mg
![2-[(4-METHOXYBENZOYL)AMINO]BENZOIC ACID](/CAS/GIF/34425-86-0.gif)
34425-86-0
13 suppliers
inquiry
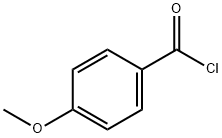
100-07-2
389 suppliers
$35.00/25g
![2-[(4-METHOXYBENZOYL)AMINO]BENZOIC ACID](/CAS/GIF/34425-86-0.gif)
34425-86-0
13 suppliers
inquiry