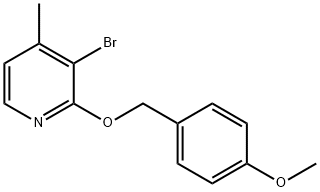
2-(4-methoxybenzyloxy)-3-bromo-4-methylpyridine synthesis
- Product Name:2-(4-methoxybenzyloxy)-3-bromo-4-methylpyridine
- CAS Number:1003312-15-9
- Molecular formula:C14H14BrNO2
- Molecular Weight:308.17
Yield:1003312-15-9 90%
Reaction Conditions:
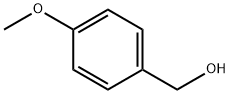
Stage #1: 4-Methoxybenzyl alcoholwith sodium hydride in tetrahydrofuran at 20; for 0.5 h;
Stage #2: 3-bromo-2-chloro-4-methylpyridine in tetrahydrofuran at 75; for 6 h;Sealed tube;
Steps:
7
3-Bromo-2-(4-methoxybenzyloxy)-4-methylpyridine
3-bromo-2-(4-methoxybenzyloxy)-4-methylpyridine was prepared by the procedure described in J. Med. Chem., 2008, 51, 3065.
A pressure vessel was charged with anhydrous THF (25 ml) and sodium hydride (1.44 g, 36.18 mmol, 60% dispersion).
To this stirred mixture was added portionwise a solution of 4-methoxybenzyl alcohol (5.0 g, 36.18 mmol) in anhydrous THF (15 ml).
After addition was complete, the mixture was stirred at room temperature for 30 minutes and a solution of 3-bromo-2-chloro-4-picoline (4.97 g, 24.08 mmol) in anhydrous THF (15 ml) was added.
The vessel was sealed and the reaction mixture was heated at 75° C. for 6 hours.
Upon cooling to room temperature, the reaction mixture was partitioned between ethyl acetate and water.
The separated organic layer was washed with water, sat'd NaCl(aq.), dried over MgSO4, filtered, and concentrated.
Elution through a flash column (silica gel 60, 230-400 mesh, 4:1 hexanes:EtOAc) gave the title compound as a clear oil which crystallized on standing (6.71 g, 90%).
References:
US2016/96832,2016,A1 Location in patent:Paragraph 0203; 0204
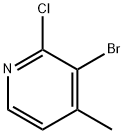
55404-31-4
151 suppliers
$9.00/1g

1003312-15-9
1 suppliers
inquiry