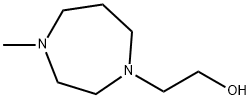
2-(4-Methyl-[1,4]diazepan-1-yl)-ethanol synthesis
- Product Name:2-(4-Methyl-[1,4]diazepan-1-yl)-ethanol
- CAS Number:59039-64-4
- Molecular formula:C8H18N2O
- Molecular Weight:158.24
4318-37-0
232 suppliers
$6.00/100mg

540-51-2
232 suppliers
$17.00/5g
![2-(4-Methyl-[1,4]diazepan-1-yl)-ethanol](/CAS/20120318/GIF/CB12578524.gif)
59039-64-4
13 suppliers
$45.00/10mg
Yield:59039-64-4 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20 - 100;
Steps:
33 2-(4-Methyl-1,4-diazepan-1-yl)ethyl 4-(4-methylphenyl)piperazine-1-carboxylate
Example 33 2-(4-Methyl-1,4-diazepan-1-yl)ethyl 4-(4-methylphenyl)piperazine-1-carboxylate 1-Methylhomopiperazine (2.00 g, 17.5 mmol) and DIPEA (3.0 mL, 18.4 mmol) were dissolved in DMF (25 mL). 2-bromethanol (1.3 mL, 18.4 mmol) was added slowly over 5 minutes. The reaction mixture was stirred at 100° C. for 2 hours and then at room temperature for 48 hours and then concentrated in vacuo. The residue was dissolved in EtOAc (-300 mL) and then washed sequentially with 1M aq Na2CO3 solution (5*200 mL), dried (MgSO4) and concentrated in vacuo to give 2-(4-methylhomopiperazin-1-yl)ethanol (2.77 g, 100%) as a brown oil which was used without further purification. Analytical LCMS: (System C, RT=0.33 min), ES+: 159.2 [MH]+. 2-(4-methylhomopiperazin-1-yl)ethanol from the previous step (2.77 g, 17.5 mmol) was dissolved in DCM (25 mL) and cooled to 0° C. NMM (2.00 mL, 18.4 mmol) and p-nitrophenyl chloroformate (3.71 g, 18.4 mmol) were added. The reaction mixture was stirred at 0° C. for 30 minutes and then at room temperature for 2 hours. A solution of 4-(4-methylphenyl)piperazine dihydrochloride (2.84 g, 11.4 mmol) and DIPEA (5.50 mL, 33.3 mmol) in DMF (40 mL) was then added. The reaction mixture was stirred at room temperature for 3 h and then concentrated in vacuo. The residue was dissolved in EtOAc (300 mL) and then washed sequentially with 1 M aq Na2CO3 solution (5*200 mL), dried (MgSO4) and concentrated in vacuo. The residue was purified by normal phase column chromatography (eluding with DCM, followed by a 85:15 mixture of DCM:MeOH) followed by reverse phase HPLC (Advanced Chromatography Technologies ACE-122-1030 RP silica 100*30 mm column, packed with Ace 5 C8 (5 μm), Pore Size 100 ?, 30 mL/min, gradient of actonitrile in water, with 0.1% trifluoroacetic acid in each solvent, 8-38%). The resulting residue was dissolved in DCM (50 mL) and stirred with solid K2CO3 for 20 minutes, filtered and concentrated in vacuo to give 2-(4-methyl-1,4-diazepan-1-yl)ethyl 4-(4-methylphenyl)piperazine-1-carboxylate (184 mg, 3.0%) as a pale yellow oil. Analytical HPLC: purity 98.1% (System A, RT=3.50 min), Analytical LCMS: purity 95.8% (System A, RT=3.96 min), ES+: 361.2 [MH]+; HRMS calcd for C20H32N4O2: 360.2525, found 360.2542.
References:
US2009/281087,2009,A1 Location in patent:Page/Page column 21