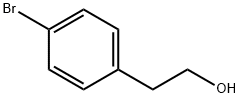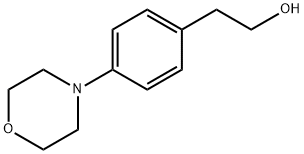
2-(4-morpholinophenyl)ethanol synthesis
- Product Name:2-(4-morpholinophenyl)ethanol
- CAS Number:105004-54-4
- Molecular formula:C12H17NO2
- Molecular Weight:207.27
Yield:105004-54-4 95%
Reaction Conditions:
with johnphos;sodium t-butanolate;palladium diacetate in toluene at 70; for 16 h;
Steps:
15 [Example 15]; (R)-3-Chloro-5,11-dihydro-5-[1-(4-morpholinophenethyl)pyrrolidin-2-yl-methyl]dibenzo[b,e][1,4]oxazepine dihydrochloride; 4-Morpholinophenethyl alcohol
A solution of 2-(4-bromophenethoxy)tetrahydro-2H-pyran (3.42 g, 12 mmol) in toluene (9.0 ml) and morpholine (1.22 g, 14 mmol) were added to a mixture of dry palladium acetate (32.3 mg, 0.14 mmol), 2-(di-t-butylphosphino)biphenyl (86.0 mg, 0.29 mmol) and sodium t-butoxide (1.73 g, 18 mmol), and they were stirred at 70°C for 16 hours. Water was added to the reaction mixture. After the extraction with ethyl acetate, the product was extracted from the organic layer with 1 M hydrochloric acid. The aqueous layer was neutralized with an aqueous sodium hydroxide solution and the product was extracted with ethyl acetate. The organic layer was dried to obtain 4-morpholinophenethyl alcohol in the form of a light yellow solid (2.35 g, 95 %). NMR (CDCl 3 ) δ : 2.80 (2H, t, J=8.7Hz) , 3.13 (4H, t, J=6.8) , 3.78-3.88(6H. m), 6.88(2H, d, J=11.7Hz), 7.14 (2H, d, J=11.7Hz)
References:
EP1403258,2004,A1 Location in patent:Page 29

![2H-Pyran, 2-[2-(4-bromophenyl)ethoxy]tetrahydro-](/CAS/20180601/GIF/79849-46-0.gif)