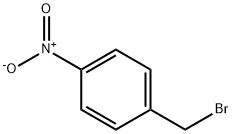
2-[(4-NITROBENZYL)OXY]-1H-ISOINDOLE-1,3(2H)-DIONE synthesis
- Product Name:2-[(4-NITROBENZYL)OXY]-1H-ISOINDOLE-1,3(2H)-DIONE
- CAS Number:30777-85-6
- Molecular formula:C15H10N2O5
- Molecular Weight:298.25
Yield:30777-85-6 97%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran; for 24 h;Heating / reflux;
Steps:
I
iV-(4-Nitrobenzyloxy)phthalimide (18); A magnetically stirred solution of 7V-hydroxyphthalimide (16) (11.2 g, 68.7 mmol) in THF (200 mL) was treated with 4-nitrobenzylbromide (17) (13.5 g, 62.5 mmol) and NJV- diisopropylethylamine (21.8 mL) and the resulting mixture heated at reflux for 24 h. The reaction mixture was then cooled and the solvent removed under reduced pressure. The ensuing residue was partitioned between H2O (150 mL) and CH2Cl2 (150 mL), the organic phase separated and the aqueous phase extracted with CH2Cl2 (1 x 100 mL). The combined organic fractions were dried (MgSO4), filtered and concentrated under reduced pressure
References:
WO2008/124878,2008,A1 Location in patent:Page/Page column 41-42
85-44-9
751 suppliers
$9.00/5g
![2-[(4-NITROBENZYL)OXY]-1H-ISOINDOLE-1,3(2H)-DIONE](/CAS/GIF/30777-85-6.gif)
30777-85-6
23 suppliers
inquiry