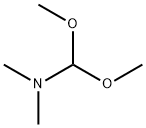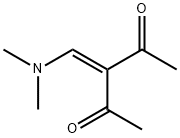
2,4-Pentanedione, 3-[(dimethylamino)methylene]- synthesis
- Product Name:2,4-Pentanedione, 3-[(dimethylamino)methylene]-
- CAS Number:18856-72-9
- Molecular formula:C8H13NO2
- Molecular Weight:155.19
Yield:18856-72-9 87%
Reaction Conditions:
in benzene at 20; for 25 h;Reflux;
Steps:
Synthesis of enaminodiones 5a-i (general procedure).
General procedure: Dimethylformamide dimethyl acetal (15.49 g, 0.13 mol) was added to 1,3-diketone 4 (0.1 mol) in anhydrous benzene (46 mL), the reaction mixture was stirred for 24 h at room temperature and then refluxed for 1 h, using a reflux condenser equipped witha calcium chloride drying tube. Then the solvent and an excess of DMF DMA were removed at reduced pressure, the residue was recrystallized from a proper solvent.
References:
Obydennov;Goncharov;Sosnovskikh, V. Ya. [Russian Chemical Bulletin,2016,vol. 65,# 9,p. 2233 - 2242][Izv. Akad. Nauk, Ser. Khim.,2016,# 9,p. 2233 - 2242]
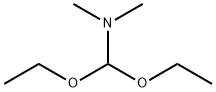
1188-33-6
249 suppliers
$11.19/5ML
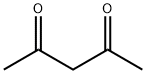
123-54-6
569 suppliers
$10.00/25ml
![2,4-Pentanedione, 3-[(dimethylamino)methylene]-](/CAS/GIF/18856-72-9.gif)
18856-72-9
7 suppliers
inquiry
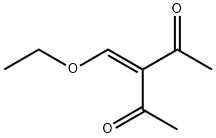
33884-41-2
31 suppliers
$66.89/5g

124-40-3
498 suppliers
$18.00/100ml
![2,4-Pentanedione, 3-[(dimethylamino)methylene]-](/CAS/GIF/18856-72-9.gif)
18856-72-9
7 suppliers
inquiry