
2-[4-(phenylsulfonyl)piperazino]-1-ethanol synthesis
- Product Name:2-[4-(phenylsulfonyl)piperazino]-1-ethanol
- CAS Number:16017-63-3
- Molecular formula:C12H18N2O3S
- Molecular Weight:270.35

412293-98-2
16 suppliers
$23.00/100mg

540-51-2
232 suppliers
$17.00/5g
![2-[4-(phenylsulfonyl)piperazino]-1-ethanol](/CAS/GIF/16017-63-3.gif)
16017-63-3
27 suppliers
$32.00/100mg
Yield:16017-63-3 88%
Reaction Conditions:
Stage #1: 2-bromoethanolwith potassium carbonate in acetonitrile at 20; for 0.5 h;
Stage #2: 1-(phenylsulfonyl)piperazine hydrochloride in acetonitrile;Reflux;
Steps:
9.4 Synthesis of 2-(4-(phenylsulfonyl)piperazin-l -yl)ethan-l -ol
To a stirred solution of 2-bromoethan-l-ol (13.2 mL, 179.6 mmol, 5.0 eq) in acetonitrile (81 mL) was added potassium carbonate (14.8 g, 107.7 mmol, 3.0 eq). The reaction mixture was stirred for about 30 minutes at room temperature then added 1- (phenylsulfonyl)piperazine.HCl salt (step 3, 8.0 g, 35.9 mmol, 1.0 eq) in acetonitrile (28 mL). The reaction mixture was refluxed for overnight. TLC indicated starting material was consumed and the desired product was observed. The reaction mixture was cooled to room temperature, and filtered. Filtrate was concentrated to get the crude residue which was purified by column chromatography by using 2% MeOH in DCM as an eluent to obtain the desired product (8.5 g, yield: 88.0%) as a colour less liquid. 1H NMR (DMSO-d6, 300 MHz): δ 7.74-7.66 (m, 5H), 4.38 (t, 1H), 3.43-3.37 (t, 2H), 2.86-2.84 (m, 4H), 2.44-2.60 (m, 2H) and 2.52-2.50 (m, 4H); Mass: [M+H]+270.66 (100%).
References:
WO2017/149518,2017,A1 Location in patent:Page/Page column 38
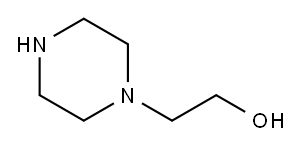
103-76-4
509 suppliers
$6.00/25g
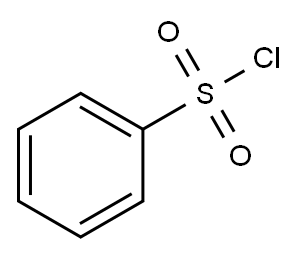
98-09-9
339 suppliers
$12.00/25g
![2-[4-(phenylsulfonyl)piperazino]-1-ethanol](/CAS/GIF/16017-63-3.gif)
16017-63-3
27 suppliers
$32.00/100mg
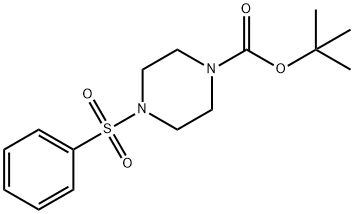
253175-69-8
6 suppliers
inquiry
![2-[4-(phenylsulfonyl)piperazino]-1-ethanol](/CAS/GIF/16017-63-3.gif)
16017-63-3
27 suppliers
$32.00/100mg