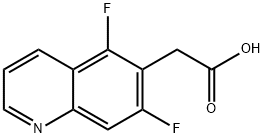
2-(5,7-difluoroquinolin-6-yl)acetic acid synthesis
- Product Name:2-(5,7-difluoroquinolin-6-yl)acetic acid
- CAS Number:1022091-46-8
- Molecular formula:C11H7F2NO2
- Molecular Weight:223.18
Yield:1022091-46-8 25%
Reaction Conditions:
Stage #1: 2-(4-amino-2,6-difluorophenyl)acetic acid;glycerolwith sulfuric acid;iron(II) sulfate in nitrobenzene at 150; for 16 h;
Stage #2: with sodium hydroxide in methanol;water;nitrobenzene at 20 - 110; for 3 h;
Stage #3: with hydrogenchloride in methanol;water;nitrobenzene; pH=3;
Steps:
32.1
Step 1: 2-(5,7-difluoroquinolin-6-yl)acetic acid A mixture of 4-amino-2,6-difluorophenylacetic acid (3.2 g, 17.1 mmol), ferrous sulphate (1.04 g, 3.76 mmol), nitrobenzene (1.05 ml, 10.26 mmol) and conc. H2SO4 in glycerol (5.2 ml) was heated at 150° C. for 16 h. The mixture was cooled to RT, methanol (28 ml) was added followed by aq. 6N NaOH (28 ml) and heated at 110° C. for 3 h. After cooling to RT, the mixture was acidified with conc. HCl to pH 3.0. The precipitate formed was filtered, washed with water and dried under vacuum. The precipitate was refluxed with methanol, filtered under hot conditions and the filtrate was evaporated to give the title compound (1 g, 25%) as a brown solid. 1H-NMR (δ ppm, DMSO-d6, 400 MHz): δ 12.72 (bs, 1H), 8.98 (d, J=3.1 Hz, 1H); 8.47 (d, J=8.4, 1H); 7.71 (d, J=9.7, 1H); 7.62 (dd, J=8.4, 4.1, 2H); 3.85 (s, 2H).
References:
US2011/281865,2011,A1 Location in patent:Page/Page column 87