
2,5,8-Undecatriyn-1-ol synthesis
- Product Name:2,5,8-Undecatriyn-1-ol
- CAS Number:35378-82-6
- Molecular formula:C11H12O
- Molecular Weight:160.21
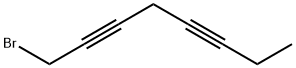
1558-79-8
10 suppliers
$460.00/100mg

107-19-7
5 suppliers
$12.00/25g

35378-82-6
7 suppliers
$425.00/50mg
Yield:35378-82-6 80%
Reaction Conditions:
with copper(l) iodide;potassium carbonate;sodium iodide in N,N-dimethyl-formamide at 20; for 12 h;Inert atmosphere;
Steps:
2,5,8-Undecatriynol (8).
A suspension of previously dried CuI (0.25 g, 1.30 mmol), NaI (0.19 g, 1.30 mmol), andK2CO3 (0.13 g, 0.97 mmol) in anhydrous DMF (3 mL) was treated with 7 (0.12 g, 0.65 mmol) and 6 (0.05 g, 0.89 mmol)dissolved in anhydrous DMF (3 mL). The system was purged with Ar, stirred for 12 h at 20°C, decomposed by saturatedNH4Cl solution (150 mL), and extracted with Et2O (5 40 mL). The organic layer was dried over Na2SO4. The solvent wasevaporated. The residue was chromatographed using a gradient of Et2O-petroleum ether (1:31:1). Yield of 8, 0.08 g (80%),Rf 0.54 (Et2O-hexane, 1:1). 1 NMR spectrum (300 MHz, CDCl3, , ppm, J/Hz): 4.27 (2H, t, J = 6.7, H-1), 3.20 (4H, m, H-4, 7),2.13, 1.32 (2H, m, H-10), 0.88 (3H, t, J = 7, H-11). 13C NMR spectrum (75 MHz, CDCl3, , ppm): 51.23 (s, -1), 77.42 (s, -2),77.32 (s, -3), 10.07 (s, -4, 7), 76.58 (s, -5, 6), 75.34 (s, -8, 9), 13.98 (s, -10), 14.88 (s, -11).
References:
Golovanov;Ivanov;Groza;Myagkova [Chemistry of Natural Compounds,2015,vol. 51,# 6,p. 1038 - 1041][Khim. Prir. Soedin.,2015,vol. 6,p. 895 - 898,4]
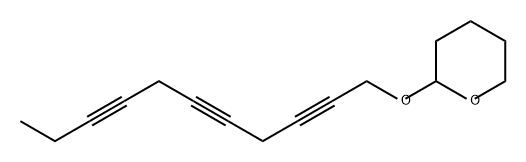
97526-03-9
0 suppliers
inquiry

35378-82-6
7 suppliers
$425.00/50mg
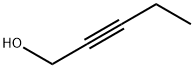
6261-22-9
208 suppliers
$17.39/1g

35378-82-6
7 suppliers
$425.00/50mg
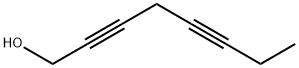
35378-76-8
9 suppliers
$120.00/10mg

35378-82-6
7 suppliers
$425.00/50mg

16400-32-1
138 suppliers
$12.00/250mg

35378-82-6
7 suppliers
$425.00/50mg