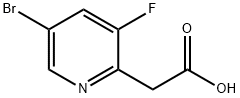
2-(5-Bromo-3-fluoropyridin-2-yl)acetic acid synthesis
- Product Name:2-(5-Bromo-3-fluoropyridin-2-yl)acetic acid
- CAS Number:1211518-85-2
- Molecular formula:C7H5BrFNO2
- Molecular Weight:234.02
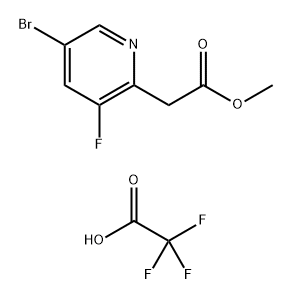
1267856-56-3
0 suppliers
inquiry

1211518-85-2
4 suppliers
inquiry
Yield:1211518-85-2 82%
Reaction Conditions:
Stage #1: methyl 2-(5-bromo-3-fluoropyridin-2-yl)acetate trifluoroacetatewith water;sodium hydroxide in methanol at 0 - 20;
Stage #2: with hydrogenchloride in water;
Steps:
148.3
To a solution of 5-bromo-3-fluoro-2-(2-methoxy-2- oxoethyl)pyridinium 2,2,2-trifluoroacetate (300 mg, 1.209 mmol) in MeOH (5 mL) at 0 °C was added aq 1M NaOH (2.41 mL, 2.41 mmol). The reaction mixture was stirred at rt overnight. The solvents were evaporated under reduced pressure and the residue was dissolved in water and acidified with IN HC1. The resulting mixture was extracted with a mixture of DCM and methanol to afford 2-(5-bromo-3-fluoropyridin- 2-yl)acetic acid (235 mg, 82%). JH NMR (300 MHz, DMSO-ifc) 12.73 (s, 1H), 8.53 (s, 1H), 8.19 (dd, J= 1.5, 9.2 Hz, 1H), 3.81 (d, J= 2.3 Hz, 2H). LC-MS (ESI) m/z 234, 236 (M+H)+.
References:
WO2011/22473,2011,A1 Location in patent:Page/Page column 237