
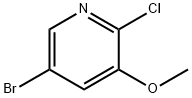
2-(5-broMo-3-Methoxypyridin-2-yl)acetonitrile synthesis
- Product Name:2-(5-broMo-3-Methoxypyridin-2-yl)acetonitrile
- CAS Number:947688-87-1
- Molecular formula:C8H7BrN2O
- Molecular Weight:227.06
286947-03-3
136 suppliers
$9.00/100mg
75-05-8
967 suppliers
$10.00/10g

947688-87-1
6 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran at 18 - 25;
Steps:
7
The 2-[5-(6,7-dimethoxyquinazolin-4-yloxy)-3-methoxypyridin-2-yl]acetic acid used as a starting material was prepared as follows :-Under an atmosphere of argon, a IM solution of lithium hexamethyldisilazane in THF (80 ml) was added dropwise to a stirred mixture of 5-bromo-2-chloro-3-methoxypyridine (International Patent Application WO 01/81347, within Example 10 thereof; 8 g), acetonitrile (4.1 ml) and THF (80 ml) that had been cooled to 18°C. Additional acetonitrile (4.1 ml) and IM lithium hexamethyldisilazane solution (80 ml) were added in turn. The reaction mixture was allowed to warm to ambient temperature and poured into a stirred mixture of water (300 ml) and diethyl ether (300 ml). The aqueous phase was extracted with diethyl ether. The
References:
WO2007/99317,2007,A1 Location in patent:Page/Page column 109-110