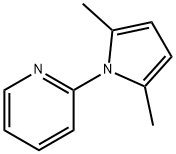
2,5-Dimethyl-1-(2-pyridinyl)-1H-pyrrole synthesis
- Product Name:2,5-Dimethyl-1-(2-pyridinyl)-1H-pyrrole
- CAS Number:32570-88-0
- Molecular formula:C11H12N2
- Molecular Weight:172.23
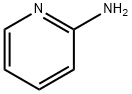
504-29-0
539 suppliers
$5.00/5G

110-13-4
378 suppliers
$10.00/5g

32570-88-0
29 suppliers
$45.00/25mg
Yield:32570-88-0 98%
Reaction Conditions:
with bentonite in neat (no solvent) at 20; for 0.0666667 h;Milling;Green chemistry;Paal-Knorr Pyrrole Synthesis;Reagent/catalyst;
Steps:
General procedure
General procedure: 2,5-hexanedione (10 mmol) and 1 g smectite clay was ground together in a mortar using pestlefor 2 min. Amine (10 mmol) was added to this mixture and grinding was continued at room temperature for the time presented in Table 1 to complete the reaction (monitored by TLC, nhexane/ ethyl acetate, 1/3). After completing the reaction the product was extracted with CH2Cl2 (2×15 mL) and the clay filtered off. The organic layer after washing with water, was dried over MgSO4, filtered and the solvent was evaporated under vacuum to afford the product. The products were isolated as low melting crystals or oils. The solid pyrroles were washed thoroughly with water, dried, and then recrystallized from methanol. The oily products were purified by column chromatography using hexane and ethyl acetate as the eluent. The solid clay portion was washed with ethanol and dried at 100 °C under a reduced pressure to be reused in thesubsequent reactions which showed the gradual decrease in the activity (Table 1). Isolated products were characterized by the melting points, IR, 1H NMR spectrometric data and werecompared with the literature or authentic samples.
References:
Marvi, Omid;Nahzomi, Hossein Taherpour [Bulletin of the Chemical Society of Ethiopia,2018,vol. 32,# 1,p. 139 - 147]

504-29-0
539 suppliers
$5.00/5G
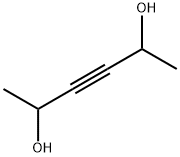
3031-66-1
124 suppliers
$10.00/1g

32570-88-0
29 suppliers
$45.00/25mg

110-13-4
378 suppliers
$10.00/5g

504-29-0
539 suppliers
$5.00/5G

3031-66-1
124 suppliers
$10.00/1g
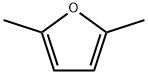
625-86-5
193 suppliers
$20.00/25mL

32570-88-0
29 suppliers
$45.00/25mg

110-13-4
378 suppliers
$10.00/5g